Induction of IDO1 and Kynurenine by Serine Proteases Subtilisin, Prostate Specific Antigen, CD26 and HtrA: A New Form of Immunosuppression?
- PMID: 35371018
- PMCID: PMC8964980
- DOI: 10.3389/fimmu.2022.832989
Induction of IDO1 and Kynurenine by Serine Proteases Subtilisin, Prostate Specific Antigen, CD26 and HtrA: A New Form of Immunosuppression?
Abstract
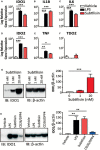
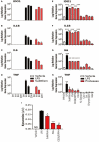
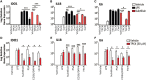
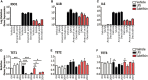
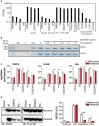
Several serine proteases have been linked to autoimmune disorders and tumour initiation although the mechanisms are not fully understood. Activation of the kynurenine pathway enzyme indoleamine-2,3-dioxygenase (IDO1) modulates cellular activity in the brain, tolerogenesis in the immune system and is a major checkpoint in cancer development. We now report that IDO1 mRNA and IDO1 protein expression (generating kynurenine) are induced in human monocyte-derived macrophages by several chymotryptic serine proteases with direct links to tumorigenesis, including Prostate Specific Antigen (PSA), CD26 (Dipeptidyl-peptidase-4, CD26/DPP-4), High Temperature Requirement protein-A (HtrA), and the bacterial virulence factor subtilisin. These proteases also induce expression of the pro-inflammatory cytokine genes IL1B and IL6. Other serine proteases tested: bacterial glu-C endopeptidase and mammalian Pro-protein Convertase Subtilase-Kexin-3 (PCSK3, furin), urokinase plasminogen activator (uPA), cathepsin G or neutrophil elastase, did not induce IDO1, indicating that the reported effects are not a general property of all serine proteases. The results represent a novel mechanism of activating immunosuppressive IDO1 and inducing kynurenine generation which, together with the production of inflammatory cytokines, would contribute to tumour initiation and progression, providing a new target for drug development. In addition, the proteasomal S20 serine protease inhibitor carfilzomib, used in the treatment of myeloma, prevented the induction of IDO1 and cytokine gene expression, potentially contributing to its clinical anti-cancer activity.
Keywords: CD26; DPP4; HtrA; IDO1; PSA; Prostate Specific Antigen; kynurenine; serine proteases.
Copyright © 2022 Clanchy, Huang, Ogbechi, Darlington, Williams and Stone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cribbs AP, Kennedy A, Penn H, Read JE, Amjadi P, Green P, et al. . Treg Cell Function in Rheumatoid Arthritis is Compromised by CTLA-4 Promoter Methylation Resulting in a Failure to Activate the Indoleamine 2,3-Dioxygenase Pathway. Arthritis Rheumatol (2014) 66:2344–54. doi: 10.1002/art.38715 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

