Poxvirus MVA Expressing SARS-CoV-2 S Protein Induces Robust Immunity and Protects Rhesus Macaques From SARS-CoV-2
- PMID: 35371043
- PMCID: PMC8966779
- DOI: 10.3389/fimmu.2022.845887
Poxvirus MVA Expressing SARS-CoV-2 S Protein Induces Robust Immunity and Protects Rhesus Macaques From SARS-CoV-2
Abstract
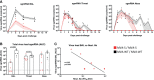
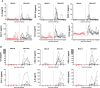
Novel safe, immunogenic, and effective vaccines are needed to control the COVID-19 pandemic, caused by SARS-CoV-2. Here, we describe the safety, robust immunogenicity, and potent efficacy elicited in rhesus macaques by a modified vaccinia virus Ankara (MVA) vector expressing a full-length SARS-CoV-2 spike (S) protein (MVA-S). MVA-S vaccination was well tolerated and induced S and receptor-binding domain (RBD)-binding IgG antibodies and neutralizing antibodies against SARS-CoV-2 and several variants of concern. S-specific IFNγ, but not IL-4, -producing cells were also elicited. After SARS-CoV-2 challenge, vaccinated animals showed a significant strong reduction of virus loads in bronchoalveolar lavages (BAL) and decreased levels in throat and nasal mucosa. Remarkably, MVA-S also protected macaques from fever and infection-induced cytokine storm. Computed tomography and histological examination of the lungs showed reduced lung pathology in MVA-S-vaccinated animals. These findings favor the use of MVA-S as a potential vaccine for SARS-CoV-2 in clinical trials.
Keywords: COVID-19; MVA vaccine; SARS-CoV-2; efficacy; immunogenicity; rhesus macaques; safety; spike.
Copyright © 2022 Mooij, García-Arriaza, Pérez, Lázaro-Frías, Verstrepen, Böszörményi, Mortier, Fagrouch, Kiemenyi-Kayere, Niphuis, Acar, Meijer, Stammes, Kondova, Verschoor, GeurtsvanKessel, de Bruin, Sikkema, Luczkowiak, Delgado, Montenegro, Puentes, Rodríguez, Bogers, Koopman and Esteban.
Conflict of interest statement
Author EP was employed by Biofabri. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. . Safety and Immunogenicity of the ChAdOx1 Ncov-19 Vaccine Against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet (2020) 396(10249):467–78. doi: 10.1016/S0140-6736(20)31604-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

