Crystal structures of 3-halo-2-organochalcogenylbenzo[ b]chalcogenophenes
- PMID: 35371552
- PMCID: PMC8900512
- DOI: 10.1107/S2056989022000962
Crystal structures of 3-halo-2-organochalcogenylbenzo[ b]chalcogenophenes
Abstract
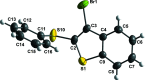
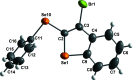
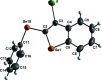
The structure of the title compounds 3-bromo-2-(phenyl-sulfan-yl)benzo[b]thiophene (C14H9BrS2; 1), 3-iodo-2-(phenyl-sulfan-yl)benzo[b]thio-phene (C14H9IS2; 2), 3-bromo-2-(phenyl-selan-yl)benzo[b]seleno-phene (C14H9BrSe2; 3), and 3-iodo-2-(phenyl-selan-yl)benzo[b]seleno-phene (C14H9ISe2; 4) were determined by single-crystal X-ray diffraction; all structures presented monoclinic (P21/c) symmetry. The phenyl group is distant from the halogen atom to minimize the steric hindrance repulsion for all structures. Moreover, the structures of 3 and 4 show an almost linear alignment of halogen-selenium-carbon atoms arising from the intra-molecular orbital inter-action between a lone pair of electrons on the halogen atom and the anti-bonding σ*Se-C orbital (n halogen→σ*Se-C). This inter-action leads to significant differences in the three-dimensional packing of the mol-ecules, which are assembled through π-π and C-H⋯π inter-actions. These data provide a better comprehension of the inter-molecular packing in benzo[b]chalcogenophenes, which is relevant for optoelectronic applications.
Keywords: benzo[b]chalcogenophenes; crystal structure; intramolecular orbital interaction.
© Q. Luz et al. 2022.
Figures





References
-
- An, Y., Oh, J., Chen, S., Lee, B., Lee, S. M., Han, D. & Yang, C. (2018). Polym. Chem. 9, 593–602.
-
- Arsenyan, P., Paegle, E., Belyakov, S., Shestakova, I., Jaschenko, E., Domracheva, I. & Popelis, J. (2011). Eur. J. Med. Chem. 46, 3434–3443. - PubMed
-
- Arsenyan, P., Petrenko, A., Leitonas, K., Volyniuk, D., Simokaitiene, J., Klinavičius, T., Skuodis, E., Lee, J.-H. & Gražulevičius, J. V. (2019). Inorg. Chem. 58, 10174–10183. - PubMed
-
- Ashraf, R. S., Meager, I., Nikolka, M., Kirkus, M., Planells, M., Schroeder, B. C., Holliday, S., Hurhangee, M., Nielsen, C. B., Sirringhaus, H. & McCulloch, L. (2015). J. Am. Chem. Soc. 137, 1314–1321. - PubMed
-
- Bondi, A. (1964). J. Phys. Chem. 68, 441–451.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
