Aging Impairs the Cellular Interplay between Myeloid Cells and Mesenchymal Cells during Skin Healing in Mice
- PMID: 35371611
- PMCID: PMC8947831
- DOI: 10.14336/AD.2021.1008
Aging Impairs the Cellular Interplay between Myeloid Cells and Mesenchymal Cells during Skin Healing in Mice
Abstract
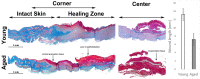
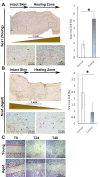
Impaired wound healing is a major issue in the elderly population and is associated with substantial health and economic burden, which is exponentially increasing with the growing aging population. While the underlying pathobiology of disturbed skin healing by aging is linked to several genetic and epigenetic factors, little is known about the cell-cell interaction during the wound healing process in aged individuals, particularly the mesenchymal stem cell (MSCs)-macrophages axis. In this study, by using a thermal injury animal model in which we compared the wound healing process of adult and young mice, we found that the insufficient pool of MSCs in adult animals are deficient in migrating to the wound bed and instead are restricted to the wound edge. We identified a deficiency of a CD90-positive MSC subpopulation in the wounds of adult animals, which is positively correlated with the number of F4/80+ macrophages. In vitro, we found that CD90+ cells preferentially adhere to the myeloid cells forming doublet cells. Thus, our findings highlight that in adult mice subjected to a thermal injury, impaired wound healing is likely mediated by a disturbed cellular interplay between myeloid cells and mesenchymal cells.
Keywords: burns; elderly; macrophages; mesenchymal stem cells; thermal injury; wound healing.
Copyright: © 2022 Amini-Nik et al.
Figures



References
-
- Bringham PA, McLoughlin E (1996). Burn incidence and medical care use in the United States: estimates, trends and data sources. J Burn Care Rehabil, 17:95-107. - PubMed
-
- (WHO) OWH. 2002. A graphical overview of the global burden of injuries. The Injury Chart Book. Geneva.
Grants and funding
LinkOut - more resources
Full Text Sources
