Knockdown of Annexin A2 Enhances Radiosensitivity by Increasing G2/M-Phase Arrest, Apoptosis and Activating the p38 MAPK-HSP27 Pathway in Nasopharyngeal Carcinoma
- PMID: 35371986
- PMCID: PMC8968728
- DOI: 10.3389/fonc.2022.769544
Knockdown of Annexin A2 Enhances Radiosensitivity by Increasing G2/M-Phase Arrest, Apoptosis and Activating the p38 MAPK-HSP27 Pathway in Nasopharyngeal Carcinoma
Abstract
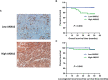
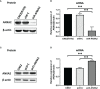
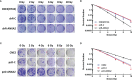
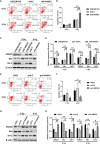
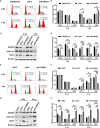
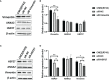
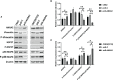
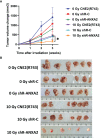
Annexin A2 (ANXA2) has been found to be involved in cancer proliferation, metastasis and prognosis; however, its exact role in nasopharyngeal carcinoma (NPC) radioresistance remains unknown. We found that ANXA2 expression was correlated with prognosis in NPC patients, and longer overall survival in NPC patients with low ANXA2 expression than those with high ANXA2 expression. ANXA2 knockdown increased the radiosensitivity in radioresistant NPC cells, and ANXA2 overexpression decreased the radiosensitivity in NPC cells. Knocking-down ANXA2 expression increased the irradiation-induced apoptosis of radioresistant NPC cells, and ANXA2 overexpression decreased the irradiation-induced apoptosis of NPC cells. ANXA2 knockdown induced G2/M phase arrest in NPC cells post-irradiation, and ANXA2 overexpression abrogated G2/M phase arrest in NPC cells post-irradiation. ANXA2 overexpression resulted in inhibition of the p38 MAPK-HSP27 pathway, while ANXA2 knockdown resulted in activation of the p38 MAPK-HSP27 pathway. In addition, ANXA2 knockdown increased the radiosensitivity of the xenografted tumors in nude mice. Our data demonstrate that knockdown of Annexin A2 enhanced radiosensitivity in NPC by increasing G2/M-phase arrest, apoptosis and activating the p38 MAPK-HSP27 pathway. ANXA2 may be a promising target used to overcome radioresistance in NPC.
Keywords: annexin A2; apoptosis; cell-cycle arrest; nasopharyngeal carcinoma; radioresistance.
Copyright © 2022 He, Lin, Zou, Pan, Fu, Zhou, Lin, Chen and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Knockdown of annexin VII enhances nasopharyngeal carcinoma cell radiosensitivity in vivo and in vitro.Cancer Biomark. 2020;28(2):129-139. doi: 10.3233/CBM-190739. Cancer Biomark. 2020. PMID: 31958076
-
Targeting annexin A2 reduces tumorigenesis and therapeutic resistance of nasopharyngeal carcinoma.Oncotarget. 2015 Sep 29;6(29):26946-59. doi: 10.18632/oncotarget.4521. Oncotarget. 2015. PMID: 26196246 Free PMC article.
-
Overexpression of β-Catenin Decreases the Radiosensitivity of Human Nasopharyngeal Carcinoma CNE-2 Cells.Cell Physiol Biochem. 2018;50(5):1929-1944. doi: 10.1159/000494873. Epub 2018 Nov 5. Cell Physiol Biochem. 2018. PMID: 30396174
-
Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma.J Biomed Sci. 2018 Mar 29;25(1):30. doi: 10.1186/s12929-018-0430-8. J Biomed Sci. 2018. PMID: 29598816 Free PMC article. Review.
-
Annexin A2 in Tumors of the Gastrointestinal Tract, Liver, and Pancreas.Cancers (Basel). 2024 Nov 8;16(22):3764. doi: 10.3390/cancers16223764. Cancers (Basel). 2024. PMID: 39594718 Free PMC article. Review.
Cited by
-
The Novel Direct AR Target Gene Annexin A2 Mediates Androgen-Induced Cellular Senescence in Prostate Cancer Cells.Biochem Genet. 2024 Nov 19. doi: 10.1007/s10528-024-10953-9. Online ahead of print. Biochem Genet. 2024. PMID: 39562436
-
Evodiamine inhibits proliferation and induces apoptosis of nasopharyngeal carcinoma cells via the SRC/ERBB2-mediated MAPK/ERK signaling pathway.J Transl Med. 2024 Sep 27;22(1):859. doi: 10.1186/s12967-024-05656-z. J Transl Med. 2024. PMID: 39334374 Free PMC article.
-
Downregulation of complement factor H attenuates the stemness of MDA‑MB‑231 breast cancer cells via modulation of the ERK and p38 signaling pathways.Oncol Lett. 2023 Oct 18;26(6):521. doi: 10.3892/ol.2023.14107. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 37927420 Free PMC article.
-
NUSAP1 promotes gastric cancer radioresistance by inhibiting ubiquitination of ANXA2 and is suppressed by miR-129-5p.J Cancer Res Clin Oncol. 2024 Aug 30;150(8):406. doi: 10.1007/s00432-024-05927-8. J Cancer Res Clin Oncol. 2024. PMID: 39212774 Free PMC article.
-
Optimizing Radiotherapy Timing for Nasopharyngeal Carcinoma: The Impact of Radiation Scheduling on Survival.JCO Precis Oncol. 2025 Jan;9:e2400603. doi: 10.1200/PO-24-00603. Epub 2025 Jan 7. JCO Precis Oncol. 2025. PMID: 39772833 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

