Exploration of the Potential Mechanism of Qi Yin San Liang San Decoction in the Treatment of EGFRI-Related Adverse Skin Reactions Using Network Pharmacology and In Vitro Experiments
- PMID: 35372072
- PMCID: PMC8964498
- DOI: 10.3389/fonc.2022.790713
Exploration of the Potential Mechanism of Qi Yin San Liang San Decoction in the Treatment of EGFRI-Related Adverse Skin Reactions Using Network Pharmacology and In Vitro Experiments
Abstract
Background: Adverse skin reactions are the most common side effects of epidermal growth factor receptor inhibitors (EGFRIs) in the treatment of cancer, significantly affecting the survival rate and quality of life of patients. Qi Yin San Liang San Decoction (QYSLS) comes from folk prescription and is currently used in the clinical treatment of adverse skin reactions caused by EGFRIs. However, its therapeutic mechanism remains unclear.
Objectives: To explore the potential mechanism of QYSLS in the treatment of adverse skin reactions caused by EGFR inhibition using network pharmacology and experimental research.
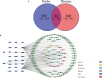
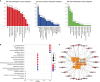
Methods: First, we verified the effectiveness of QYSLS in vivo using model mice. Second, the related targets of adverse skin reactions associated with EGFR inhibition were predicted by the Gene Expression Omnibus (GEO) database, and effective components and predictive targets of QYSLS were analyzed by Traditional Chinese Medicine Systems Pharmacology (TCMSP) and Batman-TCM databases. Gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses were performed via the Bioconductor (R) V3.8 bioinformatics software. Molecular docking studies verified the selected key ingredients and targets. Finally, the results of network pharmacology were verified by in vitro experiments.
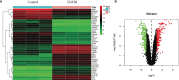
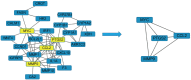
Results: In the in vivo mouse model, QYSLS effectively reduced the occurrence of skin side effects. Network pharmacological results showed that the active ingredient luteolin, quercetin, licochalcone a, and kaempferol and the effective targets prostaglandin-endoperoxide synthase 2 (PTGS2), matrix metallopeptidase 9 (MMP9), and C-C motif chemokine ligand 2 (CCL2) were related to the interleukin-17 (IL-17) and tumor necrosis factor (TNF) pathway. Subsequently, the related active compounds and targets were verified using HaCaT cells as an in vitro adverse reaction model. The results showed that luteolin and quercetin increased the expression of PTGS2 and MMP9 and reduced the expression of CCL2 in HaCaT cells treated with gefitinib.
Conclusions: The results revealed that QYSLS effectively treats EGFRI-related adverse skin reactions through multi-target and multi-pathway mechanisms. Luteolin and quercetin may be the core active ingredients of QYSLS in the treatment of EGFRI-related adverse skin reactions, and their therapeutic effects are potentially mediated through PTGS2, CCL2, and MMP9 in the IL-17 and TNF signaling pathway.
Keywords: EGFR inhibitor; QYSLS; network pharmacology; skin adverse reaction; traditional Chinese medicine.
Copyright © 2022 Wang, Zhang, Ding, Jia, Zhang, Peng, Cheng, Chen, Tan, Wang, Liu, Wei, Wang, Jiang and Hua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

