Gut Microbiota and Metabolome Description of Antibiotic-Treated Neonates From Parturients With Intrauterine Infection
- PMID: 35372104
- PMCID: PMC8974630
- DOI: 10.3389/fcimb.2022.817832
Gut Microbiota and Metabolome Description of Antibiotic-Treated Neonates From Parturients With Intrauterine Infection
Abstract
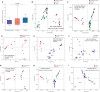
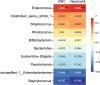
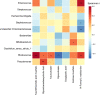
Intrauterine infection is linked to adverse pregnancy outcomes in pregnant women. Neonates from parturients with intrauterine infection are usually treated with antibiotics, but their gut microbiota and metabolome are seldom studied. In this study, we collected fecal samples from antibiotic-treated neonates of parturients with intrauterine infection (intrauterine infection group), parturients with non-intrauterine infection (antibiotic group), and untreated neonates of healthy parturients (control group). 16S rRNA gene sequencing and untargeted metabolomics analyses were performed. Our results revealed that the α-diversity of intrauterine infection group differed from that of control group. There were significant differences in β-diversity between intrauterine infection group and control group, between antibiotic group and the control group, but there was no difference between the intrauterine infection and antibiotic groups, implying that antibiotic use has an obvious effect on β-diversity and that the effects of intrauterine infection on β-diversity cannot be identified. Enterococcus was more abundant in intrauterine infection and antibiotic groups than in control group. Gut metabolite differences in intrauterine infection group and antibiotic group (only in negative ion mode) from control group were observed, but no difference between intrauterine infection group and antibiotic group was observed. N-formyl-L-methionine was the most discriminant metabolite between intrauterine infection group and control group. Primary and secondary bile acid biosynthesis, bile secretion, and cholesterol metabolism pathways were altered, and the abundances of bile acids and bile salts were altered in intrauterine infection group compared with control group. Alterations in cholesterol metabolism, arginine biosynthesis and bile secretion pathways were observed both in intrauterine infection and antibiotic groups, which might be caused by the use of antibiotics. In conclusion, we provided a preliminary description of the gut microbiota and gut metabolites in antibiotics-treated neonates from intrauterine infection parturients. Our findings did not show intrauterine infection has a separate role in neonatal gut microbiota dysbiosis, while supporting the idea that antibiotics should be used with caution during neonatal therapy.
Keywords: antibiotic; gut microbiota; intrauterine infection; metabolome; neonate.
Copyright © 2022 Li, Fu, Chen, Xu, Jing, Yang, Wan and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins.Gut. 2022 Dec;71(12):2451-2462. doi: 10.1136/gutjnl-2021-326482. Epub 2022 Apr 6. Gut. 2022. PMID: 35387876 Free PMC article.
-
Response of gut microbiota to serum metabolome changes in intrahepatic cholestasis of pregnant patients.World J Gastroenterol. 2020 Dec 14;26(46):7338-7351. doi: 10.3748/wjg.v26.i46.7338. World J Gastroenterol. 2020. PMID: 33362388 Free PMC article.
-
Perinatal Antibiotic Exposure Affects the Transmission between Maternal and Neonatal Microbiota and Is Associated with Early-Onset Sepsis.mSphere. 2020 Feb 19;5(1):e00984-19. doi: 10.1128/mSphere.00984-19. mSphere. 2020. PMID: 32075882 Free PMC article.
-
Landscapes of maternal and neonatal gut microbiome and plasma metabolome signatures and their interaction in gestational diabetes mellitus.J Nutr Biochem. 2024 Dec;134:109716. doi: 10.1016/j.jnutbio.2024.109716. Epub 2024 Aug 13. J Nutr Biochem. 2024. PMID: 39147246
-
Age-related alterations in metabolome and microbiome provide insights in dietary transition in giant pandas.mSystems. 2023 Jun 29;8(3):e0025223. doi: 10.1128/msystems.00252-23. Epub 2023 Jun 5. mSystems. 2023. PMID: 37273228 Free PMC article.
Cited by
-
Altered heme metabolism and hemoglobin concentration due to empirical antibiotics-induced gut dysbiosis in preterm infants.Comput Struct Biotechnol J. 2025 Mar 5;27:937-945. doi: 10.1016/j.csbj.2025.03.009. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40123796 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

