Matrix Metalloproteinase 2 and 9 Enzymatic Activities are Selectively Increased in the Myocardium of Chronic Chagas Disease Cardiomyopathy Patients: Role of TIMPs
- PMID: 35372112
- PMCID: PMC8968914
- DOI: 10.3389/fcimb.2022.836242
Matrix Metalloproteinase 2 and 9 Enzymatic Activities are Selectively Increased in the Myocardium of Chronic Chagas Disease Cardiomyopathy Patients: Role of TIMPs
Abstract
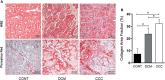
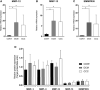
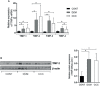
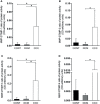
Chronic Chagas disease (CCC) is an inflammatory dilated cardiomyopathy with a worse prognosis compared to other cardiomyopathies. We show the expression and activity of Matrix Metalloproteinases (MMP) and of their inhibitors TIMP (tissue inhibitor of metalloproteinases) in myocardial samples of end stage CCC, idiopathic dilated cardiomyopathy (DCM) patients, and from organ donors. Our results showed significantly increased mRNA expression of several MMPs, several TIMPs and EMMPRIN in CCC and DCM samples. MMP-2 and TIMP-2 protein levels were significantly elevated in both sample groups, while MMP-9 protein level was exclusively increased in CCC. MMPs 2 and 9 activities were also exclusively increased in CCC. Results suggest that the balance between proteins that inhibit the MMP-2 and 9 is shifted toward their activation. Inflammation-induced increases in MMP-2 and 9 activity and expression associated with imbalanced TIMP regulation could be related to a more extensive heart remodeling and poorer prognosis in CCC patients.
Keywords: Chagas disease; MMP; cardiac remodeling; cardiomyopathy; fibrosis; heart failure; metalloproteinases.
Copyright © 2022 Baron, Ferreira, Teixeira, Moretti, Santos, Frade, Kuramoto, Debbas, Benvenuti, Gaiotto, Bacal, Pomerantzeff, Chevillard, Kalil and Cunha-Neto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy.Circulation. 1998 May 5;97(17):1708-15. doi: 10.1161/01.cir.97.17.1708. Circulation. 1998. PMID: 9591765
-
Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation.Eur J Heart Fail. 1999 Dec;1(4):337-52. doi: 10.1016/s1388-9842(99)00048-3. Eur J Heart Fail. 1999. PMID: 10937947
-
Galectin-3 (Gal-3) and the tissue inhibitor of matrix metalloproteinase (TIMP-2) as potential biomarkers for the clinical evolution of chronic Chagas cardiomyopathy.Acta Trop. 2024 Apr;252:107153. doi: 10.1016/j.actatropica.2024.107153. Epub 2024 Feb 17. Acta Trop. 2024. PMID: 38373528
-
Synergic and antagonistic relationship between MMP-2 and MMP-9 with fibrosis and inflammation in Chagas' cardiomyopathy.Parasite Immunol. 2017 Aug;39(8). doi: 10.1111/pim.12446. Epub 2017 Jun 16. Parasite Immunol. 2017. PMID: 28543409 Review.
-
Myocardial remodeling in viral heart disease: possible interactions between inflammatory mediators and MMP-TIMP system.Heart Fail Rev. 2004 Jan;9(1):21-31. doi: 10.1023/B:HREV.0000011391.81676.3c. Heart Fail Rev. 2004. PMID: 14739765 Review.
Cited by
-
Cytokine Networks as Targets for Preventing and Controlling Chagas Heart Disease.Pathogens. 2023 Jan 21;12(2):171. doi: 10.3390/pathogens12020171. Pathogens. 2023. PMID: 36839443 Free PMC article. Review.
-
Genetic Ablation and Pharmacological Blockade of Bradykinin B1 Receptor Unveiled a Detrimental Role for the Kinin System in Chagas Disease Cardiomyopathy.J Clin Med. 2023 Apr 15;12(8):2888. doi: 10.3390/jcm12082888. J Clin Med. 2023. PMID: 37109224 Free PMC article.
-
Monocyte-derived extracellular vesicles, stimulated by Trypanosoma cruzi, enhance cellular invasion in vitro via activated TGF-β1.J Extracell Vesicles. 2024 Nov;13(11):e70014. doi: 10.1002/jev2.70014. J Extracell Vesicles. 2024. PMID: 39611395 Free PMC article.
-
Advances in the Understanding and Treatment of Chronic Chagas Cardiomyopathy.Cardiol Res. 2024 Oct;15(5):340-349. doi: 10.14740/cr1665. Epub 2024 Sep 16. Cardiol Res. 2024. PMID: 39420972 Free PMC article. Review.
-
NKRF in Cardiac Fibroblasts Protects against Cardiac Remodeling Post-Myocardial Infarction via Human Antigen R.Adv Sci (Weinh). 2023 Oct;10(30):e2303283. doi: 10.1002/advs.202303283. Epub 2023 Sep 5. Adv Sci (Weinh). 2023. PMID: 37667861 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

