Association Between Gut Microbiota and Osteoarthritis: A Review of Evidence for Potential Mechanisms and Therapeutics
- PMID: 35372125
- PMCID: PMC8966131
- DOI: 10.3389/fcimb.2022.812596
Association Between Gut Microbiota and Osteoarthritis: A Review of Evidence for Potential Mechanisms and Therapeutics
Abstract
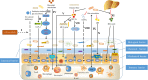
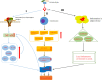
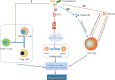
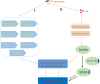
Osteoarthritis (OA) is a multifactorial joint disease characterized by degeneration of articular cartilage, which leads to joints pain, disability and reduced quality of life in patients with OA. Interpreting the potential mechanisms underlying OA pathogenesis is crucial to the development of new disease modifying treatments. Although multiple factors contribute to the initiation and progression of OA, gut microbiota has gradually been regarded as an important pathogenic factor in the development of OA. Gut microbiota can be regarded as a multifunctional "organ", closely related to a series of immune, metabolic and neurological functions. This review summarized research evidences supporting the correlation between gut microbiota and OA, and interpreted the potential mechanisms underlying the correlation from four aspects: immune system, metabolism, gut-brain axis and gut microbiota modulation. Future research should focus on whether there are specific gut microbiota composition or even specific pathogens and the corresponding signaling pathways that contribute to the initiation and progression of OA, and validate the potential of targeting gut microbiota for the treatment of patients with OA.
Keywords: gut microbiota; gut-brain axis; immune system; metabolism; osteoarthritis.
Copyright © 2022 Wei, Li and Pi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

