Altered Gut Microbiota in H1-Antihistamine-Resistant Chronic Spontaneous Urticaria Associates With Systemic Inflammation
- PMID: 35372130
- PMCID: PMC8967245
- DOI: 10.3389/fcimb.2022.831489
Altered Gut Microbiota in H1-Antihistamine-Resistant Chronic Spontaneous Urticaria Associates With Systemic Inflammation
Abstract
Background and objective: Chronic spontaneous urticaria (CSU) is a histamine-mediated inflammatory skin disease, and second-generation non-sedating H1-antihistamines (nsAH) at licensed doses have long been the first-line therapy in CSU. However, about 50% of patients are resistant to nsAH, and the precise pathogenesis remains largely unknown but seems to be associated with low-level systemic or intestinal inflammation. We aim to determine the fecal microbial composition and clarify its correlation with the clinical profiles og CSU with nsAH resistance.
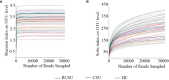
Methods: A total of 25 CSU patients with or 19 CSU patients without nsAH resistance and 19 healthy controls (HC) were enrolled in this study. The intestinal microbiome was detected by 16S rRNA sequencing. The data were analyzed using R language software.
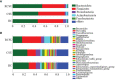
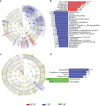
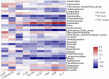
Results: Significantly higher urticarial activity score for 7 days, stool calprotectin, erythrocyte sedimentation rate, serum C-reactive protein, and interleukin-6, but much lower alpha-diversity and evenness of fecal bacterial community were observed in CSU patients with nsAH resistance than in those without (P <0.05 for all variables). Compared to patients with nsAH-responsiveness, the abundance of fecal genera Prevotella, Megamonas, and Escherichia were significantly increased, while that of Blautia, Alistipes, Anaerostipes, and Lachnospira were remarkably reduced in nsAH-resistant patients (uncorrected P <0.05 for all variables). Finally, systemic not intestinal inflammation degree was positively correlated with genera Escherichia, while negatively with genera Blautia, Dorea, Lactobacillus, Eubacterium_hallii_group, and Roseburia. CSU without nsAH resistance and HC individuals showed almost unchanged genera bacterium.
Conclusions: Among CSU patients, pro-inflammation phenotype relating to enteric dysbacteriosis features nsAH resistance in CSU patients. The results provide clues for future microbial-based or anti-inflammatory therapies on nsAH resistant CSU.
Keywords: 16S rRNA sequencing; antihistamine resistance; chronic spontaneous urticaria (CSU); gut microbiota; inflammation.
Copyright © 2022 Song, Dan, Yao, Yang, Chen and Hao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Current insights on gut microbiome and chronic urticaria: progress in the pathogenesis and opportunities for novel therapeutic approaches.Gut Microbes. 2024 Jan-Dec;16(1):2382774. doi: 10.1080/19490976.2024.2382774. Epub 2024 Jul 30. Gut Microbes. 2024. PMID: 39078229 Free PMC article. Review.
-
Lachnospira is a signature of antihistamine efficacy in chronic spontaneous urticaria.Exp Dermatol. 2022 Feb;31(2):242-247. doi: 10.1111/exd.14460. Epub 2021 Sep 30. Exp Dermatol. 2022. PMID: 34558729
-
Combined microbiome and metabolome analysis of gut microbiota and metabolite interactions in chronic spontaneous urticaria.Front Cell Infect Microbiol. 2023 Jan 11;12:1094737. doi: 10.3389/fcimb.2022.1094737. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36710970 Free PMC article.
-
Gut Microbiome Alterations and Functional Prediction in Chronic Spontaneous Urticaria Patients.J Microbiol Biotechnol. 2021 May 28;31(5):747-755. doi: 10.4014/jmb.2012.12022. J Microbiol Biotechnol. 2021. PMID: 33746191 Free PMC article.
-
Current and emerging pharmacotherapy for chronic spontaneous Urticaria: a focus on non-biological therapeutics.Expert Opin Pharmacother. 2021 Mar;22(4):497-509. doi: 10.1080/14656566.2020.1829593. Epub 2021 Feb 22. Expert Opin Pharmacother. 2021. PMID: 32990110 Review.
Cited by
-
Dysbiosis of the gut microbiota as a susceptibility factor for Kawasaki disease.Front Immunol. 2023 Oct 31;14:1268453. doi: 10.3389/fimmu.2023.1268453. eCollection 2023. Front Immunol. 2023. PMID: 38022552 Free PMC article.
-
Current insights on gut microbiome and chronic urticaria: progress in the pathogenesis and opportunities for novel therapeutic approaches.Gut Microbes. 2024 Jan-Dec;16(1):2382774. doi: 10.1080/19490976.2024.2382774. Epub 2024 Jul 30. Gut Microbes. 2024. PMID: 39078229 Free PMC article. Review.
-
Systemic inflammation is associated with gut microbiota diversity in post-stroke patients.Eur Geriatr Med. 2025 Apr;16(2):689-699. doi: 10.1007/s41999-025-01159-2. Epub 2025 Feb 11. Eur Geriatr Med. 2025. PMID: 39934474
-
Altered gut microbiota and its metabolites correlate with plasma cytokines in schizophrenia inpatients with aggression.BMC Psychiatry. 2022 Sep 27;22(1):629. doi: 10.1186/s12888-022-04255-w. BMC Psychiatry. 2022. PMID: 36167540 Free PMC article.
-
Gut microbiota facilitate chronic spontaneous urticaria.Nat Commun. 2024 Jan 2;15(1):112. doi: 10.1038/s41467-023-44373-x. Nat Commun. 2024. PMID: 38168034 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

