Surfactant Administration Through Laryngeal or Supraglottic Airways (SALSA): A Viable Method for Low-Income and Middle-Income Countries
- PMID: 35372140
- PMCID: PMC8966228
- DOI: 10.3389/fped.2022.853831
Surfactant Administration Through Laryngeal or Supraglottic Airways (SALSA): A Viable Method for Low-Income and Middle-Income Countries
Abstract
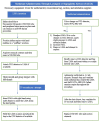
Administration of liquid surfactant through an endotracheal tube for the treatment of respiratory distress syndrome has been the standard of care for decades. A skilled health care provider is needed to perform this procedure. In lower-income and middle-income countries (LMICs), healthcare resources are often limited, leading to increased mortality of premature infants, many of whom would benefit from surfactant administration. Therefore, having a simplified procedure for delivery of surfactant without the need for advanced skills could be life-saving, potentially diminish gaps in care, and help ensure more equitable global neonatal survival rates. Modifications to the standard approach of surfactant administration have been put into practice and these include: INtubation-SURfactant-Extubation (INSURE), thin catheter surfactant administration (TCA), aerosolized surfactant, and surfactant administration through laryngeal or supraglottic airways (SALSA). Although there is a need for larger studies to evaluate the comparative effectiveness of these newer methods, these methods are being embraced by the global community and being implemented in various settings throughout the world. Because the SALSA technique does not require laryngoscopy, a provider skilled in laryngoscopy is not required for the procedure. Therefore, because of the ease of use and safety profile, the SALSA technique should be strongly considered as a viable method of delivering surfactant in LMICs.
Keywords: LISA; LMA; RDS; SALSA; aerosolized surfactant; global health; low- and middle-income countries (LMIC); surfactant.
Copyright © 2022 Zapata, Fort, Roberts, Kaluarachchi and Guthrie.
Conflict of interest statement
PF, KR, and SG have worked for and received payments from ONY Biotech in the past. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AP declared past co-authorships with one of the authors KR and the absence of any ongoing collaboration with any of the authors to the handling editor.
Figures


References
-
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. (2007) 2007:CD003063. 10.1002/14651858.CD003063.pub3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

