A Timely Administration of Antenatal Steroids Is Highly Protective Against Intraventricular Hemorrhage: An Observational Multicenter Cohort Study of Very Low Birth Weight Infants
- PMID: 35372176
- PMCID: PMC8965892
- DOI: 10.3389/fped.2022.721355
A Timely Administration of Antenatal Steroids Is Highly Protective Against Intraventricular Hemorrhage: An Observational Multicenter Cohort Study of Very Low Birth Weight Infants
Abstract
Aim: The aim of the study is to evaluate the influence of the timing of antenatal steroids (ANSs) on neonatal outcome of very low birth weight infants (VLBWI) born before 30 weeks of gestation in the German Neonatal Network.
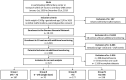
Methods: The German Neonatal Network is a large population-based cohort study enrolling VLBWIs since 2009. We included 672 neonates, who were born between January 1, 2009 and December 31, 2019 in our analysis in 10 selected centers. Infants were divided into four subgroups based on the interval between the first steroid administration and preterm birth: (I) two doses of betamethasone, ANS-birth interval: >24 h to 7 days, n = 187, (II) only one dose of betamethasone, ANS-birth interval 0-24 h, n = 70, (III) two doses of betamethasone, ANS-birth interval >7 days, n = 177, and (IV) no antenatal steroids, n = 238. Descriptive statistics and logistic regression analyses were performed for the main neonatal outcome parameters. Group IV (no ANS) was used as a reference.
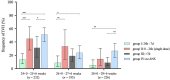
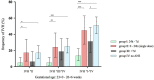
Results: An ANS-birth interval of 24 h to 7 days after the first dose was associated with a reduced risk for intraventricular hemorrhage (OR 0.17; 95% CI 0.09-0.31, p < 0.001) and mechanical ventilation (OR 0.37; 95% CI 0.23-0.61, p < 0.001), whereas the group of infants that only received a single dose of steroids reflected a subgroup at high risk for adverse neonatal outcomes; an ANS-birth interval of >7 days was still associated with a lower risk for intraventricular hemorrhage (OR 0.43; 95% CI 0.25-0.72, p = 0.002) and the need for mechanical ventilation (OR 0.43; 95% CI 0.27-0.71, p = 0.001).
Conclusion: Our observational data indicate that an ANS-birth interval of 24 h to 7 days is strongly associated with a reduced risk of intraventricular hemorrhage in VLBWIs. Further research is needed to improve the prediction of preterm birth in order to achieve a timely administration of antenatal steroids that may improve neonatal outcomes such as intraventricular hemorrhage.
Keywords: VLBWI; antenatal steroids; intraventricular hemorrhage; neonatal outcome; preterm birth.
Copyright © 2022 Fortmann, Mertens, Boeckel, Grüttner, Humberg, Astiz, Roll, Rickleffs, Rody, Härtel, Herting, Göpel and Bossung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. 10.1016/S0140-6736(12)60820-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources

