An Efficient Approach for the Design and Synthesis of Antimicrobial Peptide-Peptide Nucleic Acid Conjugates
- PMID: 35372270
- PMCID: PMC8964499
- DOI: 10.3389/fchem.2022.843163
An Efficient Approach for the Design and Synthesis of Antimicrobial Peptide-Peptide Nucleic Acid Conjugates
Abstract
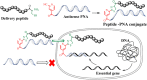
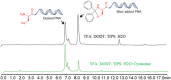
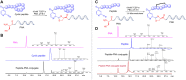
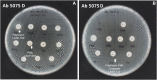
Peptide-Peptide Nucleic Acid (PNA) conjugates targeting essential bacterial genes have shown significant potential in developing novel antisense antimicrobials. The majority of efforts in this area are focused on identifying different PNA targets and the selection of peptides to deliver the peptide-PNA conjugates to Gram-negative bacteria. Notably, the selection of a linkage strategy to form peptide-PNA conjugate plays an important role in the effective delivery of PNAs. Recently, a unique Cysteine- 2-Cyanoisonicotinamide (Cys-CINA) click chemistry has been employed for the synthesis of cyclic peptides. Considering the high selectivity of this chemistry, we investigated the efficiency of Cys-CINA conjugation to synthesize novel antimicrobial peptide-PNA conjugates. The PNA targeting acyl carrier protein gene (acpP), when conjugated to the membrane-active antimicrobial peptides (polymyxin), showed improvement in antimicrobial activity against multidrug-resistant Gram-negative Acinetobacter baumannii. Thus, indicating that the Cys-CINA conjugation is an effective strategy to link the antisense oligonucleotides with antimicrobial peptides. Therefore, the Cys-CINA conjugation opens an exciting prospect for antimicrobial drug development.
Keywords: antimicrobial agents; antisense oligonucleotides; cell-penetrating peptides; conjugation; peptide nucleic acids.
Copyright © 2022 Patil, Thombare, Li, He, Lu, Yu, Wickremasinghe, Pamulapati, Azad, Velkov, Roberts and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A systematic review of peptide nucleic acids (PNAs) with antibacterial activities: Efficacy, potential and challenges.Int J Antimicrob Agents. 2024 Mar;63(3):107083. doi: 10.1016/j.ijantimicag.2024.107083. Epub 2024 Jan 5. Int J Antimicrob Agents. 2024. PMID: 38185398
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
High-Throughput Tiling of Essential mRNAs Increases Potency of Antisense Antibiotics.Adv Sci (Weinh). 2025 Jul;12(28):e2504284. doi: 10.1002/advs.202504284. Epub 2025 Apr 30. Adv Sci (Weinh). 2025. PMID: 40304263 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Topical antimicrobial agents for treating foot ulcers in people with diabetes.Cochrane Database Syst Rev. 2017 Jun 14;6(6):CD011038. doi: 10.1002/14651858.CD011038.pub2. Cochrane Database Syst Rev. 2017. PMID: 28613416 Free PMC article.
Cited by
-
Peptide nucleic acid conjugates and their antimicrobial applications-a mini-review.Eur Biophys J. 2023 Oct;52(6-7):533-544. doi: 10.1007/s00249-023-01673-w. Epub 2023 Aug 23. Eur Biophys J. 2023. PMID: 37610696 Free PMC article. Review.
-
Chemical strategies for antisense antibiotics.Chem Soc Rev. 2024 Nov 25;53(23):11303-11320. doi: 10.1039/d4cs00238e. Chem Soc Rev. 2024. PMID: 39436264 Free PMC article. Review.
-
Dataset on substituents effect on biological activities of linear RGD-containing peptides as potential anti-angiotensin converting enzyme.Data Brief. 2023 Aug 6;50:109478. doi: 10.1016/j.dib.2023.109478. eCollection 2023 Oct. Data Brief. 2023. PMID: 37600591 Free PMC article.
-
Iron uptake pathway of Escherichia coli as an entry route for peptide nucleic acids conjugated with a siderophore mimic.Front Microbiol. 2024 Jan 31;15:1331021. doi: 10.3389/fmicb.2024.1331021. eCollection 2024. Front Microbiol. 2024. PMID: 38357356 Free PMC article.
-
Cell-Free Systems: Ideal Platforms for Accelerating the Discovery and Production of Peptide-Based Antibiotics.Int J Mol Sci. 2024 Aug 22;25(16):9109. doi: 10.3390/ijms25169109. Int J Mol Sci. 2024. PMID: 39201795 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

