Acid-Base Equilibrium and Dielectric Environment Regulate Charge in Supramolecular Nanofibers
- PMID: 35372273
- PMCID: PMC8965714
- DOI: 10.3389/fchem.2022.852164
Acid-Base Equilibrium and Dielectric Environment Regulate Charge in Supramolecular Nanofibers
Abstract
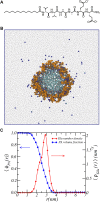
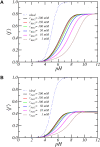
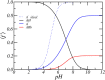
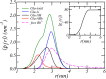
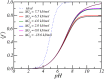
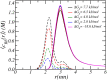
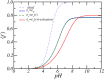
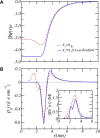
Peptide amphiphiles are a class of molecules that can self-assemble into a variety of supramolecular structures, including high-aspect-ratio nanofibers. It is challenging to model and predict the charges in these supramolecular nanofibers because the ionization state of the peptides are not fixed but liable to change due to the acid-base equilibrium that is coupled to the structural organization of the peptide amphiphile molecules. Here, we have developed a theoretical model to describe and predict the amount of charge found on self-assembled peptide amphiphiles as a function of pH and ion concentration. In particular, we computed the amount of charge of peptide amphiphiles nanofibers with the sequence C 16 - V 2 A 2 E 2. In our theoretical formulation, we consider charge regulation of the carboxylic acid groups, which involves the acid-base chemical equilibrium of the glutamic acid residues and the possibility of ion condensation. The charge regulation is coupled with the local dielectric environment by allowing for a varying dielectric constant that also includes a position-dependent electrostatic solvation energy for the charged species. We find that the charges on the glutamic acid residues of the peptide amphiphile nanofiber are much lower than the same functional group in aqueous solution. There is a strong coupling between the charging via the acid-base equilibrium and the local dielectric environment. Our model predicts a much lower degree of deprotonation for a position-dependent relative dielectric constant compared to a constant dielectric background. Furthermore, the shape and size of the electrostatic potential as well as the counterion distribution are quantitatively and qualitatively different. These results indicate that an accurate model of peptide amphiphile self-assembly must take into account charge regulation of acidic groups through acid-base equilibria and ion condensation, as well as coupling to the local dielectric environment.
Keywords: charge regulation; dielectric constant; ion condensation; peptide amphiphiles; theory.
Copyright © 2022 Nap, Qiao, Palmer, Stupp, Olvera de la Cruz and Szleifer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Atkins P. W. (1998). Physical Chemistry. 6th ed. New York: Freeman. edn.
-
- Avni Y., Andelman D., Podgornik R. (2019). Charge Regulation with Fixed and mobile Charged Macromolecules. Curr. Opin. Electrochem. 13, 70–77. 10.1016/j.coelec.2018.10.014 - DOI
LinkOut - more resources
Full Text Sources

