Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis
- PMID: 35372295
- PMCID: PMC8965449
- DOI: 10.3389/fbioe.2022.856591
Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis
Abstract
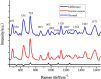
Gastric cancer is usually diagnosed at late stage and has a high mortality rate, whereas early detection of gastric cancer could bring a better prognosis. Conventional gastric cancer diagnostic methods suffer from long diagnostic times, severe trauma, and a high rate of misdiagnosis and rely heavily on doctors' subjective experience. Raman spectroscopy is a label-free molecular vibrational spectroscopy technique that identifies the molecular fingerprint of various samples based on the inelastic scattering of monochromatic light. Because of its advantages of non-destructive, rapid, and accurate detection, Raman spectroscopy has been widely studied for benign and malignant tumor differentiation, tumor subtype classification, and section pathology diagnosis. This paper reviews the applications of Raman spectroscopy for the in vivo and in vitro diagnosis of gastric cancer, methodology related to the spectroscopy data analysis, and presents the limitations of the technique.
Keywords: Raman spectroscopy; clinical diagnostics; gastric cancer; machine learning; on-site applications.
Copyright © 2022 Liu, Zhao, Li and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

