Prokaryotic Collagen-Like Proteins as Novel Biomaterials
- PMID: 35372322
- PMCID: PMC8968730
- DOI: 10.3389/fbioe.2022.840939
Prokaryotic Collagen-Like Proteins as Novel Biomaterials
Abstract
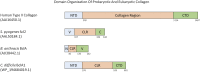
Collagens are the major structural component in animal extracellular matrices and are critical signaling molecules in various cell-matrix interactions. Its unique triple helical structure is enabled by tripeptide Gly-X-Y repeats. Understanding of sequence requirements for animal-derived collagen led to the discovery of prokaryotic collagen-like protein in the early 2000s. These prokaryotic collagen-like proteins are structurally similar to mammalian collagens in many ways. However, unlike the challenges associated with recombinant expression of mammalian collagens, these prokaryotic collagen-like proteins can be readily expressed in E. coli and are amenable to genetic modification. In this review article, we will first discuss the properties of mammalian collagen and provide a comparative analysis of mammalian collagen and prokaryotic collagen-like proteins. We will then review the use of prokaryotic collagen-like proteins to both study the biology of conventional collagen and develop a new biomaterial platform. Finally, we will describe the application of Scl2 protein, a streptococcal collagen-like protein, in thromboresistant coating for cardiovascular devices, scaffolds for bone regeneration, chronic wound dressing and matrices for cartilage regeneration.
Keywords: biomaterials; collagen; hydrogel; integrin; integrin-targeting biomaterials; prokaryotic collagen-like protein; streptococcal collagen-like protein.
Copyright © 2022 Picker, Lan, Arora, Green, Hahn, Cosgriff-Hernandez and Hook.
Conflict of interest statement
MHo and EC-H report a stakeholder interest in ECM Biosurgery which seeks to commercialize Designer Collagens based on the Scl2 protein. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

