HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer
- PMID: 35372498
- PMCID: PMC8965450
- DOI: 10.3389/fmolb.2022.834651
HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer
Abstract
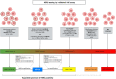
HER2 status in breast cancer is assessed to select patients eligible for targeted therapy with anti-HER2 therapies. According to the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP), the HER2 test positivity is defined by protein overexpression (score 3+) at immunohistochemistry (IHC) and/or gene amplification at in situ hybridization (ISH). The introduction of novel anti-HER2 compounds, however, is changing this paradigm because some breast cancers with lower levels of protein expression (i.e. score 1+/2+ with no gene amplification) benefited from HER2 antibody-drug conjugates (ADC). Recently, a potential for HER2 targeting in HER2 "ultra-low" (i.e. score 0 with incomplete and faint staining in ≤10% of tumor cells) and MutL-deficient estrogen receptor (estrogen receptor)-positive/HER2-negative breast cancers has been highlighted. All these novel findings are transforming the traditional dichotomy of HER2 status and have dramatically raised the expectations in this field. Still, a more aware HER2 status assessment coupled with the comprehensive characterization of the clinical and molecular features of these tumors is required. Here, we seek to provide an overview of the current state of HER2 targeting in breast cancers beyond the canonical HER2 positivity and to discuss the practical implications for pathologists and oncologists.
Keywords: HER2 low expression; HER2 ultra low; antibody-drug conjugate; biomarkers; breast cancer; fluorescence in situ hybrdization; immunohistochemistry; targeted therapy.
Copyright © 2022 Venetis, Crimini, Sajjadi, Corti, Guerini-Rocco, Viale, Curigliano, Criscitiello and Fusco.
Conflict of interest statement
GC received honoraria for consulting/advisory role/speaker bureau and/or travel funding from Roche, Lilly, Bristol-Myers Squibb, Pfizer, Novartis, and Seagen; GV from MSD Oncology, Pfizer, Dako, Roche/Genetech, Astellas Pharma, Novartis, Bayer, Daiichi; Sankyo, Menarini, Ventana Medical Systems Dako/Agilent Technologies, Cepheid, and Celgene. CC received honoraria for consulting/advisory role/speaker bureau from Novartis, Eli-Lilly, Pfizer, MSD, Seagen and Roche; NF from Merck Sharp and Dohme (MSD), Boehringer Ingelheim, Novartis, AstraZeneca, and Daiichi Sankyo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Comment in
-
Response to letter entitled: Re: "Evolution of low HER2 expression between early and advanced-stage breast cancer".Eur J Cancer. 2023 Jan;179:149-151. doi: 10.1016/j.ejca.2022.10.008. Epub 2022 Nov 9. Eur J Cancer. 2023. PMID: 36371304 No abstract available.
-
Re: "Evolution of low HER2 expression between early and advanced-stage breast cancer".Eur J Cancer. 2023 Jan;179:147-148. doi: 10.1016/j.ejca.2022.10.007. Epub 2022 Nov 13. Eur J Cancer. 2023. PMID: 36379839 No abstract available.
References
-
- Bachelot T., Ciruelos E., Schneeweiss A., Puglisi F., Peretz-Yablonski T., Bondarenko I., et al. (2019). Preliminary Safety and Efficacy of First-Line Pertuzumab Combined with Trastuzumab and Taxane Therapy for HER2-Positive Locally Recurrent or Metastatic Breast Cancer (PERUSE). Ann. Oncol. 30, 766–773. 10.1093/annonc/mdz061 - DOI - PubMed
-
- Banerji U., van Herpen C. M. L., Saura C., Thistlethwaite F., Lord S., Moreno V., et al. (2019). Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: a Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 20, 1124–1135. 10.1016/s1470-2045(19)30328-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

