Design and Characterization of Agarose/HPMC Buccal Films Bearing Ondansetron HCl In Vitro and In Vivo: Enhancement Using Iontophoretic and Chemical Approaches
- PMID: 35372569
- PMCID: PMC8975656
- DOI: 10.1155/2022/1662194
Design and Characterization of Agarose/HPMC Buccal Films Bearing Ondansetron HCl In Vitro and In Vivo: Enhancement Using Iontophoretic and Chemical Approaches
Retraction in
-
Retracted: Design and Characterization of Agarose/HPMC Buccal Films Bearing Ondansetron HCl In Vitro and In Vivo: Enhancement Using Iontophoretic and Chemical Approaches.Biomed Res Int. 2024 Jan 9;2024:9841865. doi: 10.1155/2024/9841865. eCollection 2024. Biomed Res Int. 2024. PMID: 38230163 Free PMC article.
Abstract
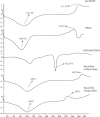
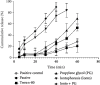
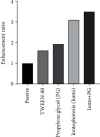
The study was aimed at designing and characterizing the ondansetron hydrochloride (OND) bearing agarose (AG), and hydroxypropyl methyl cellulose (HPMC) mucoadhesive buccal films employing glycerol as a plasticizer. The buccal delivery of ondansetron hydrochloride was remarkably boosted by employing physical (iontophoresis) and chemical enhancement approaches (chemical penetration enhancers). To explore the influence of different formulation components, i.e., agarose, hydroxypropyl methyl cellulose (HPMC), and glycerol on various evaluating parameters, i.e., tensile strength, swelling index, ex vivo mucoadhesion time, and subsequently on in vitro drug release, a D-optimal design was opted. A buccal film bearing OND was mounted on bovine buccal mucosa for ex vivo permeation studies and impact of chemical and physical enhancement techniques on the permeation profile was also analysed. A linear release profile was revealed in in vitro drug release of OND over 60 minutes and outcomes ascertained the direct relationship between HPMC content and in vitro drug release and inverse relationship was depicted by AG content. The FTIR and DSC thermal analysis was executed to determine the physicochemical interactions and results exposed no chemical interactions between drug and polymers. The drug (OND) appeared as tiny crystals on smooth film surface during scanning electron microscopy (SEM) analysis. A notable enhancement in permeation flux, i.e., 761.02 μg/min of OND during ex vivo permeation studies was witnessed after the application of current (0.5-1 mA) without any time lag and with enhancement ratio of 3.107. A time lag of 15 minutes, 19 minutes, and 26 minutes with permeation flux of 475.34 μg/min, 399.35 μg/min, and 244.81 μg/min was observed after chemical enhancer pretreatment with propylene glycol, Tween 80, and passive, respectively. Rabbit was employed as the experimental animal for pharmacokinetic studies (in vivo) and cats for pharmacological activity (in vivo), and the results illustrated the enhanced bioavailablity (2.88 times) in the iontophoresis animal group when compared with the rabbits of control group. Likewise, a remarkable reduction in emesis events was recorded in cats of iontophoresis group. Conclusively, the histopathological examinations on excised buccal mucosa unveiled no severe necrotic or cytopathetic outcomes of current.
Copyright © 2022 Umair Jillani et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Santos G. A., Doty M. S. Agarose from Gracilariacylindrica. Botanica Marina . 1983;26(1):31–34. doi: 10.1515/botm.1983.26.1.31. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

