TRUE-1: Trial of Repurposed Unithiol for snakebite Envenoming phase 1 (safety, tolerability, pharmacokinetics and pharmacodynamics in healthy Kenyan adults)
- PMID: 35372700
- PMCID: PMC8961198
- DOI: 10.12688/wellcomeopenres.17682.1
TRUE-1: Trial of Repurposed Unithiol for snakebite Envenoming phase 1 (safety, tolerability, pharmacokinetics and pharmacodynamics in healthy Kenyan adults)
Abstract
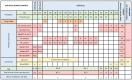
Background: Snakebites affect over 5 million people each year, and over 100,000 per year die as a result. The only available treatment is antivenom, which has many shortcomings including high cost, intravenous administration, and high risk of adverse events. One of the most abundant and harmful components of viper venoms are the zinc-dependent snake venom metalloproteinases (SVMPs). Unithiol is a chelating agent which is routinely used to treat heavy metal poisoning. In vivo experiments in small animal models have demonstrated that unithiol can prevent local tissue damage and death caused by a certain viper species. This phase I clinical trial will assess the safety of ascending doses of unithiol with a view for repurposing for snakebite indication. Methods: This open label, single agent, phase I clinical trial of a repurposed drug has a primary objective to evaluate the safety of escalating doses of unithiol, and a secondary objective to describe its pharmacokinetics. In total, 64 healthy Kenyan volunteers from Kilifi County will be dosed in consecutive groups of eight, with dose escalation decisions dependent on review of safety data by an independent data safety monitoring board. Four groups will receive ascending single oral doses, two will receive multiple oral doses, and two will receive single intravenous doses. Follow-up will be for 6-months and includes full adverse event reporting. Pharmacokinetic analysis will define the Cmax, Tmax, half-life and renal elimination. Conclusions: This clinical trial will assess the safety and tolerability of a promising oral therapeutic in a relevant setting where snakebites are prevalent. Unithiol is likely to be safer than antivenom, is easier to manufacture, has activity against diverse snake species, and can be administered orally, and thus shows promise for repurposing for tropical snakebite. Pan African Clinical Trials Registry: PACTR202103718625048 (3/3/2021).
Keywords: Snakebite; adaptive; chelator; clinical trial.; envenoming; phase I; repurpose; small molecule.
Copyright: © 2022 Abouyannis M et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

