Early Administration of Anti-SARS-CoV-2 Monoclonal Antibodies Prevents Severe COVID-19 in Kidney Transplant Patients
- PMID: 35372734
- PMCID: PMC8957354
- DOI: 10.1016/j.ekir.2022.03.020
Early Administration of Anti-SARS-CoV-2 Monoclonal Antibodies Prevents Severe COVID-19 in Kidney Transplant Patients
Abstract
Introduction: Kidney transplant recipients (KTRs) are prone to develop severe COVID-19 and are less well protected by vaccine than immunocompetent subjects. Thus, the use of neutralizing anti-SARS-CoV-2 monoclonal antibody (MoAb) to confer a passive immunity appears attractive in KTRs.
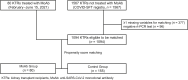
Methods: We performed a French nationwide study to compare COVID-19-related hospitalization, 30-day admission to intensive care unit (ICU), and 30-day death between KTRs who received an early infusion of MoAb (MoAb group) and KTRs who did not (control group). Controls were identified from the COVID-SFT registry (NCT04360707) using a propensity score matching with the following covariates: age, sex, delay between transplantation and infection, induction and maintenance immunosuppressive therapy, initial symptoms, and comorbidities.
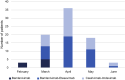
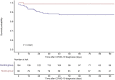
Results: A total of 80 KTRs received MoAb between February 2021 and June 2021. They were matched to 155 controls. COVID-19-related hospitalization, 30-day admission to ICU, and 30-day death were less frequently observed in the MoAb group (35.0% vs. 49.7%, P = 0.032; 2.5% vs. 15.5%, P = 0.002; 1.25% vs. 11.6%, P = 0.005, respectively). No patient required mechanical ventilation in the MoAb group. The number of patients to treat to prevent 1 death was 9.7.
Conclusion: The early use of MoAb in KTRs with a mild form of COVID-19 largely improved outcomes in KTRs.
Keywords: COVID-19; monoclonal antibody; transplantation; viral infection.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

