COVID-19 Patients Require Prolonged Extracorporeal Membrane Oxygenation Support for Survival Compared With Non-COVID-19 Patients
- PMID: 35372842
- PMCID: PMC8966959
- DOI: 10.1097/CCE.0000000000000671
COVID-19 Patients Require Prolonged Extracorporeal Membrane Oxygenation Support for Survival Compared With Non-COVID-19 Patients
Abstract
To investigate the ICU survival of venovenous extracorporeal membrane oxygenation (ECMO) patients suffering from COVID-19-related acute respiratory distress syndrome (ARDS) versus ECMO patients without COVID-19 (non-COVID-19)-related ARDS.
Design: Preliminary analysis of data from two prospective ECMO trials and retrospective analysis of a cohort of ARDS ECMO patients.
Setting: Single-center ICU.
Patients: Adult ARDS ECMO patients, 16 COVID-19 versus 23 non-COVID-19 patients. Analysis of retrospective data from 346 adult ARDS ECMO patients.
Interventions: None.
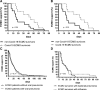
Measurements and main results: COVID-19 and non-COVID-19 ARDS patients did not differ with respect to preexisting disease or body mass index. ICU survival rate was 62% for COVID-19 ECMO patients and 70% for non-COVID-19 ECMO patients. COVID-19 ECMO survivors were supported with ECMO for a median of 43 days (interquartile range [IQR], 18-58 d) versus 16 days (IQR, 19-39 d; p = 0.03) for non-COVID-19 patients. The median duration of ECMO therapy for all ARDS patients between 2007 and 2018 was 15 days (IQR, 6-28 d). The subgroup of patients suffering from any viral pneumonia received ECMO support for a median of 16 days (IQR, 9-27 d), survivors of influenza pneumonia received ECMO support for 13 days (IQR, 7-25 d).
Conclusions: COVID-19 patients required significant longer ECMO support compared with patients without COVID-19 to achieve successful ECMO weaning and ICU survival.
Keywords: COVID-19; duration of extracorporeal membrane oxygenation therapy; extracorporeal membrane oxygenation survival; pulmonary fibrotic remodeling; venovenous extracorporeal membrane oxygenation.
Copyright © 2022 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- World Health Organization: Weekly Epidemiological Update on COVID-19. 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Accessed August 20, 2021
-
- Extracorporeal Life Support Organization: Extracorporeal Life Support Organization (ELSO) Registry. 2021. Available at: https://www.elso.org/. Accessed August 27, 2021
LinkOut - more resources
Full Text Sources

