Regional Variance of the Early Use of Tolvaptan for Autosomal Dominant Polycystic Kidney Disease
- PMID: 35372950
- PMCID: PMC8815752
- DOI: 10.34067/KID.0002262020
Regional Variance of the Early Use of Tolvaptan for Autosomal Dominant Polycystic Kidney Disease
Abstract
Background: The development and prompt dissemination of the first drug against a particular disease can contribute to improvements in national health status and medical economy end points and are assumedly affected by socioeconomic factors that have yet to be analyzed. Tolvaptan, a vasopressin receptor 2 antagonist, was developed to treat hyponatremia, congestive heart failure, and cirrhosis ascites, although the approved indications may differ among countries. In Japan, high-dose tolvaptan tablets were approved as the first drug for autosomal dominant polycystic kidney disease (ADPKD) in 2014. This study aimed to better understand the factors that influence the total number of regional prescriptions of tolvaptan for ADPKD since its launch.
Methods: The National Database of Health Insurance Claims and Specific Health Checkups of Japan Open Data was used as a national claim-based database. In each of the 47 prefectures in Japan, the total prescribed number of 30 mg tolvaptan tablets between 2015 and 2017 was examined. The parameters explaining the prescription variation among regions were then examined by correlation analysis.
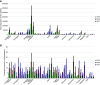
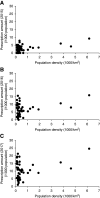
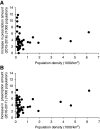
Results: Prescriptions for high-dose tolvaptan increased substantially 2 years after the drug's approval; however, the increase differed by approximately 21-fold between regions. Population density was positively associated with prescribed 30 mg tolvaptan tablets per 1000 population in 2015 (r=0.47, P<0.001). In addition, the increase in prescribed number of tablets per 1000 population was correlated with population density in 2016-2017 (r=0.30, P=0.04).
Conclusions: This macro perspective analysis revealed an urban-rural inequity in prescriptions for the newly approved drug for ADPKD. Further studies are needed to elucidate the factors affecting the geographic variation.
Keywords: Japan; autosomal dominant; cystic kidney disease; polycystic kidney; population density; regional variance; tolvaptan.
Copyright © 2020 by the American Society of Nephrology.
Figures

References
-
- Groves KE, Schellinck T, Sketris I, MacKinnon NJ: Identifying early prescribers of cycloxygenase-2 inhibitors (COX-2s) in Nova Scotia, Canada: Considerations for targeted academic detailing. Res Social Adm Pharm 6: 257–267, 2010 - PubMed
-
- Prosser H, Almond S, Walley T: Influences on GPs’ decision to prescribe new drugs-the importance of who says what. Fam Pract 20: 61–68, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

