Safety and Efficacy of Tenapanor for Long-term Serum Phosphate Control in Maintenance Dialysis: A 52-Week Randomized Phase 3 Trial (PHREEDOM)
- PMID: 35372979
- PMCID: PMC8785778
- DOI: 10.34067/KID.0002002021
Safety and Efficacy of Tenapanor for Long-term Serum Phosphate Control in Maintenance Dialysis: A 52-Week Randomized Phase 3 Trial (PHREEDOM)
Abstract
Background: Treating hyperphosphatemia is a tenet of dialysis care. This trial assessed the safety and efficacy of tenapanor for the management of hyperphosphatemia.
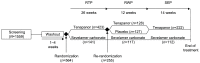
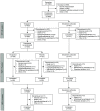
Methods: In this 52-week phase 3 study (NCT03427125), participants receiving maintenance dialysis with both hyperphosphatemia (serum phosphate 6.0-10.0 mg/dl) and a 1.5 mg/dl increase after phosphate binder washout were randomized (3:1) to tenapanor 30 mg twice daily for 26 weeks (randomized treatment period) or sevelamer carbonate (52-week safety control). Participants completing 26 weeks of treatment with tenapanor were rerandomized (1:1) to tenapanor or placebo for 12 weeks (randomized withdrawal period), and were eligible to enter the 14-week safety extension period. With input from the US Food and Drug Administration, the primary efficacy end point was the difference in the change in serum phosphate from the end of the randomized treatment period to the end of the randomized withdrawal period, among participants who achieved ≥1.2 mg/dl decrease in serum phosphate during the randomized treatment period (efficacy analysis set). Efficacy was also evaluated in the intention-to-treat (ITT) analysis set.
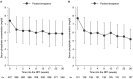
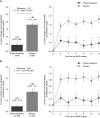
Results: Of 564 eligible participants randomized to receive tenapanor (n=423) or sevelamer carbonate (n=141) during the randomized treatment period, 255 (60%) in the tenapanor group subsequently were rerandomized to tenapanor (n=128) or placebo (n=127) during the randomized withdrawal period. In the efficacy analysis set (n=131), the difference in estimated mean change in serum phosphate level between tenapanor and placebo from the beginning to the end of the randomized withdrawal period was -1.4 mg/dl (P<0.0001); in the ITT analysis set (n=243), the estimated mean difference was -0.7 mg/dl (P=0.002). Loosened stools were the most frequently reported adverse event (53% during the randomized treatment period). Serious adverse events were reported more frequently for participants treated with sevelamer carbonate (16%-23% across the three study periods) compared with tenapanor (11%-17%).
Conclusions: Tenapanor reduced serum phosphate concentrations and maintained control of serum phosphate in participants receiving maintenance dialysis, with an acceptable safety and tolerability profile.
Keywords: FGF23; NHE3; dialysis; hyperphosphatemia; mineral metabolism; phosphate; phosphate uptake; tenapanor.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
A.J. Bleyer reports no affiliation with Ardelyx, Inc. aside from his role as Principal Investigator in the PHREEDOM study. A.L. Silva reports receiving research funding from Ardelyx, Inc. D.E. Weiner reports being the Medical Director of Clinical Research for Dialysis Clinic, Inc., with support paid to his institution by Dialysis Clinic, Inc.; he reports consulting for Akebia, Cara Therapeutics, Janssen Biopharmaceuticals, and Tricida, and has no affiliation with or received funding from Ardelyx, Inc., aside from his role as site Principal Investigator in several tenapanor trials. D.P. Rosenbaum reports being an employee of, and having an ownership interest in, Ardelyx, Inc. G.A. Block reports being a Director for Ardelyx, Inc. and is the Associate Chief Medical Officer for US Renal Care, Inc. G.M. Chertow reports being a consultant to, and has equity ownership interest in, Ardelyx, Inc. R.I. Lynn has no affiliation with Ardelyx, Inc. aside from his role as Principal Investigator in the PHREEDOM and NORMALIZE studies. Y. Yang reports being an employee of Ardelyx, Inc.
Figures

References
-
- Lopes MB, Karaboyas A, Bieber B, Pisoni RL, Walpen S, Fukagawa M, Christensson A, Evenepoel P, Pegoraro M, Robinson BM, Pecoits-Filho R: Impact of longer term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant 35: 1794–1801, 2020. 10.1093/ndt/gfaa054 - DOI - PMC - PubMed
-
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D: Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 24: 1506–1523, 2009. 10.1093/ndt/gfn613 - DOI - PubMed
-
- Arbor Research Collaborative for Health: Serum phosphorus (most recent): National sample. Available at: http://www.dopps.org/DPM. Accessed August 27, 2020
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

