In Vivo Entombment of Bacteria and Fungi during Calcium Oxalate, Brushite, and Struvite Urolithiasis
- PMID: 35373025
- PMCID: PMC8740987
- DOI: 10.34067/KID.0006942020
In Vivo Entombment of Bacteria and Fungi during Calcium Oxalate, Brushite, and Struvite Urolithiasis
Abstract
Background: Human kidney stones form via repeated events of mineral precipitation, partial dissolution, and reprecipitation, which are directly analogous to similar processes in other natural and manmade environments, where resident microbiomes strongly influence biomineralization. High-resolution microscopy and high-fidelity metagenomic (microscopy-to-omics) analyses, applicable to all forms of biomineralization, have been applied to assemble definitive evidence of in vivo microbiome entombment during urolithiasis.
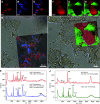
Methods: Stone fragments were collected from a randomly chosen cohort of 20 patients using standard percutaneous nephrolithotomy (PCNL). Fourier transform infrared (FTIR) spectroscopy indicated that 18 of these patients were calcium oxalate (CaOx) stone formers, whereas one patient formed each formed brushite and struvite stones. This apportionment is consistent with global stone mineralogy distributions. Stone fragments from seven of these 20 patients (five CaOx, one brushite, and one struvite) were thin sectioned and analyzed using brightfield (BF), polarization (POL), confocal, super-resolution autofluorescence (SRAF), and Raman techniques. DNA from remaining fragments, grouped according to each of the 20 patients, were analyzed with amplicon sequencing of 16S rRNA gene sequences (V1-V3, V3-V5) and internal transcribed spacer (ITS1, ITS2) regions.
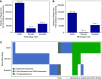
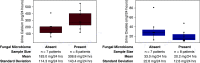
Results: Bulk-entombed DNA was sequenced from stone fragments in 11 of the 18 patients who formed CaOx stones, and the patients who formed brushite and struvite stones. These analyses confirmed the presence of an entombed low-diversity community of bacteria and fungi, including Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Aspergillus niger. Bacterial cells approximately 1 μm in diameter were also optically observed to be entombed and well preserved in amorphous hydroxyapatite spherules and fans of needle-like crystals of brushite and struvite.
Conclusions: These results indicate a microbiome is entombed during in vivo CaOx stone formation. Similar processes are implied for brushite and struvite stones. This evidence lays the groundwork for future in vitro and in vivo experimentation to determine how the microbiome may actively and/or passively influence kidney stone biomineralization.
Keywords: Raman spectroscopy; bacteria; basic science; fungi entombment; geomicrobiology; kidney stone mineralogy; microbiome; nephrolithiasis; super-resolution autofluorescence (SRAF) microscopy; urolithiasis.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
A. Krambeck reports consultancy agreements with Boston Scientific, Lumenis, Sonomotion, and Virtuoso; receiving research funding from Boston Scientific, Lumenis; receiving honoraria from Boston Scientific, Lumenis, Sonomotion, and Virtuoso; reports having patents and inventions b7h1 and Survivin as a marker for Renal Cell Carcinoma; and reports being a scientific advisor or member of Boston Scientific, Sonomotion, and Virtuoso. B. Fouke reports receiving research funding from Dornier MedTech. D. Lange reports having consultancy agreements with AdvaTec, BD/Bard, Boston Scientific, Cook Medical, and Kisolite; having an ownership interest in Kisolite Corp; reports receiving research funding from AdvaTec, BD/Bard, Boston Scientific, and Cook Medical; and reports scientific advisor or membership of Kisolite Corp. J. Lieske reports having consultancy agreements with Alnylam, Allena, American Board of Internal Medicine, Dicerna, Orfan, OxThera, Retrophin, and Siemens; reports receiving research funding from Allena, Alnylam, Dicerna, OxThera, Retrophin, and Siemens; reports receiving honoraria from Alnylam, Allena, American Board of Internal Medicine, Dicerna, Retrophin, Novobiome, Orfan, OxThera, and Synlogic; scientific advisor or membership of American Board of Internal Medicine, Hyperoxaluria Foundation, Kidney International, and Oxalosis. M. Rivera reports consultancy agreements with Boston Scientific, Cook Medical and Lumenis. M. Romero reports scientific advisor or membership of Kidney360 - Associate Editor, American Journal of Physiology-Renal Physiology, Hyperoxaluria Foundation, Oxalosis, National Institute of Diabetes and Digestive and Kidney Diseases study sections, ad hoc. N. Chia reports receiving research funding from Archer Daniels Midland. T. Large reports having consultancy agreements with Boston Scientific and Lumenis. Y. Dong reports being a scientific advisor or member of Frontiers in Microbiology. All remaining authors have nothing to disclose. All remaining authors have nothing to disclose.
Figures






References
-
- Sivaguru M, Saw JJ, Williams JC Jr, Lieske JC, Krambeck AE, Romero MF, Chia N, Schwaderer AL, Alcalde RE, Bruce WJ, Wildman DE, Fried GA, Werth CJ, Reeder RJ, Yau PM, Sanford RA, Fouke BW: Geobiology reveals how human kidney stones dissolve in vivo. Sci Rep 8: 13731, 2018. 10.1038/s41598-018-31890-9 - DOI - PMC - PubMed
-
- Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ: The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser 5: 126–136, 2007. 10.1016/j.eeus.2007.03.002 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

