Proximal Tubular Oxidative Metabolism in Acute Kidney Injury and the Transition to CKD
- PMID: 35373028
- PMCID: PMC8740982
- DOI: 10.34067/KID.0004772020
Proximal Tubular Oxidative Metabolism in Acute Kidney Injury and the Transition to CKD
Abstract
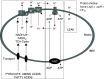
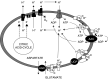
The proximal tubule relies on oxidative mitochondrial metabolism to meet its energy needs and has limited capacity for glycolysis, which makes it uniquely susceptible to damage during AKI, especially after ischemia and anoxia. Under these conditions, mitochondrial ATP production is initially decreased by several mechanisms, including fatty acid-induced uncoupling and inhibition of respiration related to changes in the shape and volume of mitochondria. Glycolysis is initially insufficient as a source of ATP to protect the cells and mitochondrial function, but supplementation of tricarboxylic acid cycle intermediates augments anaerobic ATP production, and improves recovery of mitochondrial oxidative metabolism. Incomplete recovery is characterized by defects of respiratory enzymes and lipid metabolism. During the transition to CKD, tubular cells atrophy but maintain high expression of glycolytic enzymes, and there is decreased fatty acid oxidation. These metabolic changes may be amenable to a number of therapeutic interventions.
Keywords: AKI; CKD; aTP; acute kidney injury and ICU nephrology; basic science; glycolysis; metabolism; mitochondria; tricarboxylic acid cycle.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
M.A. Venkatachalam reports honoraria from Surrozen. All remaining authors have nothing to disclose.
Figures






References
-
- Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, Parikh SM: De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. 10.1038/s41591-018-0138-z - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

