Podocyte Lipotoxicity in CKD
- PMID: 35373048
- PMCID: PMC8791311
- DOI: 10.34067/KID.0006152020
Podocyte Lipotoxicity in CKD
Abstract
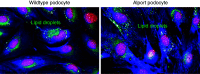
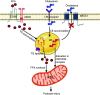
CKD represents the ninth most common cause of death in the United States but, despite this large health burden, treatment options for affected patients remain limited. To remedy this, several relevant pathways have been identified that may lead to novel therapeutic options. Among them, altered renal lipid metabolism, first described in 1982, has been recognized as a common pathway in clinical and experimental CKD of both metabolic and nonmetabolic origin. This observation has led many researchers to investigate the cause of this renal parenchyma lipid accumulation and its downstream effect on renal structure and function. Among key cellular components of the kidney parenchyma, podocytes are terminally differentiated cells that cannot be easily replaced when lost. Clinical and experimental evidence supports a role of reduced podocyte number in the progression of CKD. Given the importance of the podocytes in the maintenance of the glomerular filtration barrier and the accumulation of TG and cholesterol-rich lipid droplets in the podocyte and glomerulus in kidney diseases that cause CKD, understanding the upstream cause and downstream consequences of lipid accumulation in podocytes may lead to novel therapeutic opportunities. In this review, we hope to consolidate our understanding of the causes and consequences of dysregulated renal lipid metabolism in CKD development and progression, with a major focus on podocytes.
Keywords: basic science; chronic kidney disease; lipid accumulation; podocyte lipotoxicity.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
A. Fornoni is inventor on pending or issued patents (PCT/US11/56272, PCT/US12/62594, PCT/US2019/041730, PCT/US2019/032215, PCT/US13/36484, and PCT 62/674897) aimed at diagnosing or treating proteinuric kidney diseases. She stands to gain royalties from her future commercialization of these patents. A. Fornoni is vice president of L&F Health LLC and is a consultant for ZyVersa Therapeutics, Inc. ZyVersa Therapeutics, Inc. has licensed worldwide rights to develop and commercialize hydroxypropyl-β-cyclodextrin from L&F Research for the treatment of kidney disease. All remaining authors have nothing to disclose.
Figures
References
-
- Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V: US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States (available at https://www.usrds.org). Am J Kidney Dis 75[Suppl 1]: A6–A7, 2020. Available at: 10.1053/j.ajkd.2019.09.003 - DOI - PubMed
-
- Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, Molina J, Varona Santos J, Ge M, Sloan A, Kim JJ, Pedigo C, Bryn J, Volosenco I, Faul C, Zeidan YH, Garcia Hernandez C, Mendez AJ, Leibiger I, Burke GW, Futerman AH, Barisoni L, Ishimoto Y, Inagi R, Merscher S, Fornoni A: SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun 10: 2692, 2019. 10.1038/s41467-019-10584-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

