Functional and quality of life outcomes of localised prostate cancer treatments (Prostate Testing for Cancer and Treatment [ProtecT] study)
- PMID: 35373443
- PMCID: PMC9543725
- DOI: 10.1111/bju.15739
Functional and quality of life outcomes of localised prostate cancer treatments (Prostate Testing for Cancer and Treatment [ProtecT] study)
Abstract
Objective: To investigate the functional and quality of life (QoL) outcomes of treatments for localised prostate cancer and inform treatment decision-making.
Patients and methods: Men aged 50-69 years diagnosed with localised prostate cancer by prostate-specific antigen testing and biopsies at nine UK centres in the Prostate Testing for Cancer and Treatment (ProtecT) trial were randomised to, or chose one of, three treatments. Of 2565 participants, 1135 men received active monitoring (AM), 750 a radical prostatectomy (RP), 603 external-beam radiotherapy (EBRT) with concurrent androgen-deprivation therapy (ADT) and 77 low-dose-rate brachytherapy (BT, not a randomised treatment). Patient-reported outcome measures (PROMs) completed annually for 6 years were analysed by initial treatment and censored for subsequent treatments. Mixed effects models were adjusted for baseline characteristics using propensity scores.
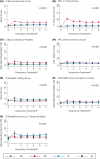
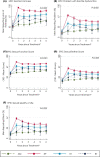
Results: Treatment-received analyses revealed different impacts of treatments over 6 years. Men remaining on AM experienced gradual declines in sexual and urinary function with age (e.g., increases in erectile dysfunction from 35% of men at baseline to 53% at 6 years and nocturia similarly from 20% to 38%). Radical treatment impacts were immediate and continued over 6 years. After RP, 95% of men reported erectile dysfunction persisting for 85% at 6 years, and after EBRT this was reported by 69% and 74%, respectively (P < 0.001 compared with AM). After RP, 36% of men reported urinary leakage requiring at least 1 pad/day, persisting for 20% at 6 years, compared with no change in men receiving EBRT or AM (P < 0.001). Worse bowel function and bother (e.g., bloody stools 6% at 6 years and faecal incontinence 10%) was experienced by men after EBRT than after RP or AM (P < 0.001) with lesser effects after BT. No treatment affected mental or physical QoL.
Conclusion: Treatment decision-making for localised prostate cancer can be informed by these 6-year functional and QoL outcomes.
Keywords: #PCSM; #ProstateCancer; #uroonc; functional outcomes; localised prostate cancer; patient-reported outcomes; quality of life; treatments.
© 2022 The Authors. BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

