Independent distribution between tauopathy secondary to subacute sclerotic panencephalitis and measles virus: An immunohistochemical analysis in autopsy cases including cases treated with aggressive antiviral therapies
- PMID: 35373453
- PMCID: PMC9616085
- DOI: 10.1111/bpa.13069
Independent distribution between tauopathy secondary to subacute sclerotic panencephalitis and measles virus: An immunohistochemical analysis in autopsy cases including cases treated with aggressive antiviral therapies
Abstract
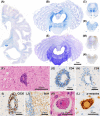
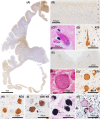
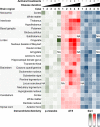
Subacute sclerotic panencephalitis (SSPE) is a refractory neurological disorder after exposure to measles virus. Recently, SSPE cases have been treated with antiviral therapies, but data on the efficacy are inconclusive. Abnormal tau accumulation has been reported in the brain tissue of SSPE cases, but there are few reports in which this is amply discussed. Five autopsied cases diagnosed as definite SSPE were included in this study. The subject age or disease duration ranged from 7.6 to 40.9 years old or from 0.5 to 20.8 years, respectively. Cases 3 and 4 had been treated with antiviral therapies. All evaluated cases showed marked brain atrophy with cerebral ventricle dilatation; additionally, marked demyelination with fibrillary gliosis were observed in the cerebral white matter. The brainstem, cerebellum, and spinal cord were relatively preserved. Immunoreactivity (IR) against measles virus was seen in the brainstem tegmentum, neocortex, and/or limbic cortex of the untreated cases but was rarely seen in the two treated cases. Activated microglia were broadly observed from the cerebrum to the spinal cord and had no meaningful difference among cases. Neurofibrillary tangles characterized by a combination of 3- and 4-repeat tau were observed mainly in the oculomotor nuclei, locus coeruleus, and limbic cortex. IR against phosphorylated tau was seen mainly in the cingulate gyrus, oculomotor nuclei, and pontine tegmentum, and tended to be observed frequently in cases with long disease durations but also tended to decrease along with neuronal loss, as in Case 5, which had the longest disease duration. Since the distribution of phosphorylated tau was independent from that of measles virus, the tauopathy following SSPE was inferred to be the result of diffuse brain inflammation triggered by measles rather than a direct result of measles virus. Moreover, antiviral therapies seemed to suppress measles virus but not the progression of tauopathy.
Keywords: autopsy; measles virus; neurofibrillary tangle; subacute sclerotic panencephalitis; tau.
© 2022 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

References
-
- Hotta H, Jiang DP, Nagano‐Fujii M. SSPE virus and pathogenesis. Nihon Rinsho Jpn J Clin Med. 2007;65(8):1475–80. - PubMed
-
- Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52(10):901–7. - PubMed
-
- Hosoya M. Anti SSPE drugs. Nihon Rinsho Jpn J Clin Med. 2012;70(4):625–8. - PubMed
-
- Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41(1):3–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

