Biallelic ADAM22 pathogenic variants cause progressive encephalopathy and infantile-onset refractory epilepsy
- PMID: 35373813
- PMCID: PMC9337806
- DOI: 10.1093/brain/awac116
Biallelic ADAM22 pathogenic variants cause progressive encephalopathy and infantile-onset refractory epilepsy
Abstract
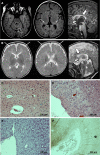
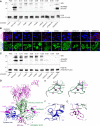
Pathogenic variants in A Disintegrin And Metalloproteinase (ADAM) 22, the postsynaptic cell membrane receptor for the glycoprotein leucine-rich repeat glioma-inactivated protein 1 (LGI1), have been recently associated with recessive developmental and epileptic encephalopathy. However, so far, only two affected individuals have been described and many features of this disorder are unknown. We refine the phenotype and report 19 additional individuals harbouring compound heterozygous or homozygous inactivating ADAM22 variants, of whom 18 had clinical data available. Additionally, we provide follow-up data from two previously reported cases. All affected individuals exhibited infantile-onset, treatment-resistant epilepsy. Additional clinical features included moderate to profound global developmental delay/intellectual disability (20/20), hypotonia (12/20) and delayed motor development (19/20). Brain MRI findings included cerebral atrophy (13/20), supported by post-mortem histological examination in patient-derived brain tissue, cerebellar vermis atrophy (5/20), and callosal hypoplasia (4/20). Functional studies in transfected cell lines confirmed the deleteriousness of all identified variants and indicated at least three distinct pathological mechanisms: (i) defective cell membrane expression; (ii) impaired LGI1-binding; and/or (iii) impaired interaction with the postsynaptic density protein PSD-95. We reveal novel clinical and molecular hallmarks of ADAM22 deficiency and provide knowledge that might inform clinical management and early diagnostics.
Keywords: ADAM22; LGI1; developmental and epileptic encephalopathy; refractory seizures.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Orsini A, Zara F, Striano P. Recent advances in epilepsy genetics. Neurosci Lett. 2018;667:4–9. - PubMed
-
- Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313(5794):1792–1795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

