Detection and identification of coronaviruses in human tissues using electron microscopy
- PMID: 35373872
- PMCID: PMC9088335
- DOI: 10.1002/jemt.24115
Detection and identification of coronaviruses in human tissues using electron microscopy
Abstract
The identification of viral particles within a tissue specimen requires specific knowledge of viral ultrastructure and replication, as well as a thorough familiarity with normal subcellular organelles. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has underscored how challenging the task of identifying coronavirus by electron microscopy (EM) can be. Numerous articles have been published mischaracterizing common subcellular structures, including clathrin- or coatomer- coated vesicles, multivesicular bodies, and rough endoplasmic reticulum, as coronavirus particles in SARS-CoV-2 positive patient tissue specimens. To counter these misinterpretations, we describe the morphological features of coronaviruses that should be used to differentiate coronavirus particles from subcellular structures. Further, as many of the misidentifications of coronavirus particles have stemmed from attempts to attribute tissue damage to direct infection by SARS-CoV-2, we review articles describing ultrastructural changes observed in specimens from SARS-CoV-2-infected individuals that do not necessarily provide EM evidence of direct viral infection. Ultrastructural changes have been observed in respiratory, cardiac, kidney, and intestinal tissues, highlighting the widespread effects that SARS-CoV-2 infection may have on the body, whether through direct viral infection or mediated by SARS-CoV-2 infection-induced inflammatory and immune processes. HIGHLIGHTS: The identification of coronavirus particles in SARS-CoV-2 positive tissues continues to be a challenging task. This review provides examples of coronavirus ultrastructure to aid in the differentiation of the virus from common cellular structures.
Keywords: COVID-19; SARS; SARS-CoV-2; coronavirus; electron microscopy; ultrastructure.
© 2022 Wiley Periodicals LLC. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures

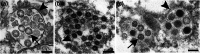
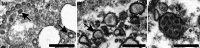
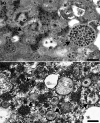
References
-
- Akilesh, S. , Nast, C. C. , Yamashita, M. , Henriksen, K. , Charu, V. , Troxell, M. L. , Kambham, N. , Bracamonte, E. , Houghton, D. , Ahmed, N. I. , Chong, C. C. , Thajudeen, B. , Rehman, S. , Khoury, F. , Zuckerman, J. E. , Gitomer, J. , Raguram, P. C. , Mujeeb, S. , Schwarze, U. , … Smith, K. D. (2021). Multicenter clinicopathologic correlation of kidney biopsies performed in COVID‐19 patients presenting with acute kidney injury or proteinuria. American Journal of Kidney Diseases, 77(1), 82–93. 10.1053/j.ajkd.2020.10.001 - DOI - PMC - PubMed
-
- Akilesh, S. , Nicosia, R. F. , Alpers, C. E. , Tretiakova, M. , Hsiang, T. Y. , Gale, M. , & Smith, K. D. (2021). Characterizing viral infection by electron microscopy: Lessons from the coronavirus disease 2019 pandemic. The American Journal of Pathology, 191(2), 222–227. 10.1016/j.ajpath.2020.11.003 - DOI - PMC - PubMed
-
- Alsaad, K. O. , Hajeer, A. H. , Al Balwi, M. , Al Moaiqel, M. , Al Oudah, N. , Al Ajlan, A. , AlJohani, S. , Alsolamy, S. , Gmati, G. E. , Balkhy, H. , Al‐Jahdali, H. H. , Baharoon, S. A. , & Arabi, Y. M. (2018). Histopathology of Middle East respiratory syndrome coronovirus (MERS‐CoV) infection – Clinicopathological and ultrastructural study. Histopathology, 72(3), 516–524. 10.1111/his.13379 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

