Intrahepatic cholestasis of pregnancy - Time to redefine the reference range of total serum bile acids: A cross-sectional study
- PMID: 35373886
- PMCID: PMC9543426
- DOI: 10.1111/1471-0528.17174
Intrahepatic cholestasis of pregnancy - Time to redefine the reference range of total serum bile acids: A cross-sectional study
Abstract
Objective: To establish pregnancy-specific reference ranges for fasting and postprandial total serum bile acid (TSBA) concentrations.
Design: Cross-sectional study.
Setting: Tertiary-care university hospital.
Population: Healthy pregnant women at term admitted to the Obstetrics Department over a period of 1 year. Exclusion criteria were an established diagnosis of intrahepatic cholestasis of pregnancy (ICP) or any coexisting condition of increased risk for ICP.
Methods: Both fasting (after 8-14 h of fasting) and postprandial (2 h after meal) TSBA concentrations were measured in 612 women (with 528 fasting samples and 377 postprandial samples) by automated enzymatic spectrophotometric assay.
Main outcome measures: Fasting and postprandial TSBA concentrations in 612 women.
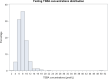
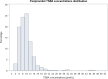
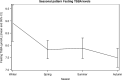
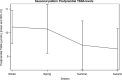
Results: Reference intervals of 4.4-14.1 μmol/L for fasting TSBA and 4.7-20.2 μmol/L for postprandial TSBA were established. The postprandial values were significantly higher than the fasting values, with a median increase of 1.0 μmol/L (p < 0.0001). A correlation between fasting TSBA concentrations and postprandial concentrations was found, as well as correlations with fetal sex, parity and assisted reproductive technologies. A seasonal pattern was noticed for both fasting and postprandial TSBA, with the highest values measured in the winter season (p < 0.01 and 0.02, respectively) CONCLUSIONS: Normal pregnancy is associated with mild hypercholanaemia, and therefore a higher threshold should be considered for the diagnosis of ICP. We suggest using the upper reference limits observed in our healthy pregnant population (14 μmol/L for fasting TSBA and 20 μmol/L for postprandial TSBA). As the fasting measurement is more specific for the diagnosis, and the postprandial measurement is essential for the assessment of severity, it is recommended to measure both values rather than use random sampling.
Tweetable abstract: Normal pregnancy is associated with mild hypercholanaemia, a higher threshold should be considered for the diagnosis of ICP.
Keywords: diagnostic thresholds; intrahepatic cholestasis of pregnancy; reference ranges; total serum bile acids.
© 2022 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared. Completed disclosure of interests form available to view online as supporting information.
Figures
References
-
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124:120–33. - PubMed
-
- Kenyon AP, Piercy CN, Girling J, Williamson C, Tribe RM, Shennan AH. Obstetric cholestasis, outcome with active management: a series of 70 cases. BJOG. 2002;109:282–8. - PubMed
-
- Dixon PH, Williamson C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol. 2016;40:141–53. - PubMed
-
- Smith DD, Rood KM. Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol. 2020;63(1):134–51. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials