Intervertebral Disc Degeneration: Biomaterials and Tissue Engineering Strategies toward Precision Medicine
- PMID: 35373924
- PMCID: PMC11469247
- DOI: 10.1002/adhm.202102530
Intervertebral Disc Degeneration: Biomaterials and Tissue Engineering Strategies toward Precision Medicine
Abstract
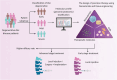
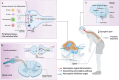
Intervertebral disc degeneration is a common cause of discogenic low back pain resulting in significant disability. Current conservative or surgical intervention treatments do not reverse the underlying disc degeneration or regenerate the disc. Biomaterial-based tissue engineering strategies exhibit the potential to regenerate the disc due to their capacity to modulate local tissue responses, maintain the disc phenotype, attain biochemical homeostasis, promote anatomical tissue repair, and provide functional mechanical support. Despite preliminary positive results in preclinical models, these approaches have limited success in clinical trials as they fail to address discogenic pain. This review gives insights into the understanding of intervertebral disc pathology, the emerging concept of precision medicine, and the rationale of personalized biomaterial-based tissue engineering tailored to the severity of the disease targeting early, mild, or severe degeneration, thereby enhancing the efficacy of the treatment for disc regeneration and ultimately to alleviate discogenic pain. Further research is required to assess the relationship between disc degeneration and lower back pain for developing future clinically relevant therapeutic interventions targeted towards the subgroup of degenerative disc disease patients.
Keywords: biomaterials; discogenic low back pain; intervertebral discs; precision medicine; tissue engineering.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vos T., Lim S. S., Abbafati C., Abbas K. M., Abbasi M., Abbasifard M., Abbasi‐Kangevari M., Abbastabar H., Abd‐Allah F., Abdelalim A., Abdollahi M., Abdollahpour I., Abolhassani H., Aboyans V., Abrams E. M., Abreu L. G., Abrigo M. R. M., Abu‐Raddad L. J., Abushouk A. I., Acebedo A., Ackerman I. N., Adabi M., Adamu A. A., Adebayo O. M., Adekanmbi V., Adelson J. D., Adetokunboh O. O., Adham D., Afshari M., Afshin A., et al., Lancet 2020, 396, 1204. - PubMed
-
- Raftery M. N., Sarma K., Murphy A. W., De L. a., Harpe D., Normand C., McGuire B. E., Pain 2011, 152, 1096. - PubMed
-
- Fullen B., Hurley D. A., Power C., Canavan D., O'Keeffe D., Ir. J. Med. Sci. 2006, 175, 68. - PubMed
-
- Hall J. A., Jowett S., Lewis M., Oppong R., Konstantinou K., Pain 2021, 162, 702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

