Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0
- PMID: 35376991
- PMCID: PMC9165250
- DOI: 10.1007/s00259-022-05780-2
Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0
Abstract
Purpose: The goal of this guideline/procedure standard is to assist nuclear medicine physicians, other nuclear medicine professionals, oncologists or other medical specialists for recommended use of [18F]FDG PET/CT in oncological patients undergoing immunotherapy, with special focus on response assessment in solid tumors.
Methods: In a cooperative effort between the EANM, the SNMMI and the ANZSNM, clinical indications, recommended imaging procedures and reporting standards have been agreed upon and summarized in this joint guideline/procedure standard.
Conclusions: The field of immuno-oncology is rapidly evolving, and this guideline/procedure standard should not be seen as definitive, but rather as a guidance document standardizing the use and interpretation of [18F]FDG PET/CT during immunotherapy. Local variations to this guideline should be taken into consideration.
Preamble: The European Association of Nuclear Medicine (EANM) is a professional non-profit medical association founded in 1985 to facilitate worldwide communication among individuals pursuing clinical and academic excellence in nuclear medicine. The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and professional organization founded in 1954 to promote science, technology and practical application of nuclear medicine. The Australian and New Zealand Society of Nuclear Medicine (ANZSNM), founded in 1969, represents the major professional society fostering the technical and professional development of nuclear medicine practice across Australia and New Zealand. It promotes excellence in the nuclear medicine profession through education, research and a commitment to the highest professional standards. EANM, SNMMI and ANZSNM members are physicians, technologists, physicists and scientists specialized in the research and clinical practice of nuclear medicine. All three societies will periodically put forth new standards/guidelines for nuclear medicine practice to help advance the science of nuclear medicine and improve service to patients. Existing standards/guidelines will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each standard/guideline, representing a policy statement by the EANM/SNMMI/ANZSNM, has undergone a thorough consensus process, entailing extensive review. These societies recognize that the safe and effective use of diagnostic nuclear medicine imaging requires particular training and skills, as described in each document. These standards/guidelines are educational tools designed to assist practitioners in providing appropriate and effective nuclear medicine care for patients. These guidelines are consensus documents based on current knowledge. They are not intended to be inflexible rules or requirements of practice, nor should they be used to establish a legal standard of care. For these reasons and those set forth below, the EANM, SNMMI and ANZSNM caution against the use of these standards/guidelines in litigation in which the clinical decisions of a practitioner are called into question. The ultimate judgment regarding the propriety of any specific procedure or course of action must be made by medical professionals considering the unique circumstances of each case. Thus, there is no implication that an action differing from what is laid out in the guidelines/procedure standards, standing alone, is below standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set forth in the standards/guidelines when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources or advances in knowledge or technology subsequent to publication of the guidelines/procedure standards. The practice of medicine involves not only the science, but also the art of dealing with the prevention, diagnosis, alleviation and treatment of disease. The variety and complexity of human conditions make it impossible for general guidelines to consistently allow for an accurate diagnosis to be reached or a particular treatment response to be predicted. Therefore, it should be recognized that adherence to these standards/ guidelines will not ensure a successful outcome. All that should be expected is that practitioners follow a reasonable course of action, based on their level of training, current knowledge, clinical practice guidelines, available resources and the needs/context of the patient being treated. The sole purpose of these guidelines is to assist practitioners in achieving this objective. The present guideline/procedure standard was developed collaboratively by the EANM, the SNMMI and the ANZSNM, with the support of international experts in the field. They summarize also the views of the Oncology and Theranostics and the Inflammation and Infection Committees of the EANM, as well as the procedure standards committee of the SNMMI, and reflect recommendations for which the EANM and SNMMI cannot be held responsible. The recommendations should be taken into the context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
Keywords: PET/CT; Positron emission tomography; [18F]FDG; guideline; immunotherapy; malignant tumors; treatment response.
© 2022. The Author(s).
Conflict of interest statement
E.L. reports receiving grants from AIRC (Associazione Italiana per la Ricerca sul Cancro) and from the Italian Ministry of Health and faculty remuneration from ESMIT (European School of Multimodality Imaging and Therapy) and MI&T congressi. R. J.H. is on the Scientific Advisory Board of Telix Pharmaceuticals with any honoraria donated to his institution, he is a stock holder in this company, and he is also an honorary Trustee of the International Cancer Imaging Society and honorary Board Member of Neuroendocrine Cancer Australia. W.A.W. has been on the advisory boards and receives compensation from Blue Earth Diagnostics. N.P. declares honoraria from Actinium Pharmaceuticals, AstraZeneca/MedImmune; Consulting or Advisory Role from Illumina, progenics; Speakers' Bureau from Actinium Pharmaceuticals; Research Funding from Bayer Health; Bristol-Myers Squibb; Clarity Pharmaceuticals; Imaginab; Janssen; Regeneron; Travel, Accommodations Expenses from Bayer. The other authors declare no conflicts of interest.
Figures

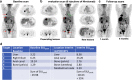
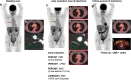
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

