Subcortical Neuronal Correlates of Sleep in Neurodegenerative Diseases
- PMID: 35377391
- PMCID: PMC8981071
- DOI: 10.1001/jamaneurol.2022.0429
Subcortical Neuronal Correlates of Sleep in Neurodegenerative Diseases
Abstract
Importance: Sleep disturbance is common among patients with neurodegenerative diseases. Examining the subcortical neuronal correlates of sleep disturbances is important to understanding the early-stage sleep neurodegenerative phenomena.
Objectives: To examine the correlation between the number of important subcortical wake-promoting neurons and clinical sleep phenotypes in patients with Alzheimer disease (AD) or progressive supranuclear palsy (PSP).
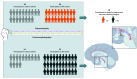
Design, setting, and participants: This longitudinal cohort study enrolled 33 patients with AD, 20 patients with PSP, and 32 healthy individuals from the Memory and Aging Center of the University of California, San Francisco, between August 22, 2008, and December 31, 2020. Participants received electroencephalographic and polysomnographic sleep assessments. Postmortem neuronal analyses of brainstem hypothalamic wake-promoting neurons were performed and were included in the clinicopathological correlation analysis. No eligible participants were excluded from the study.
Exposures: Electroencephalographic and polysomnographic assessment of sleep and postmortem immunohistological stereological analysis of 3 wake-promoting nuclei (noradrenergic locus coeruleus [LC], orexinergic lateral hypothalamic area [LHA], and histaminergic tuberomammillary nucleus [TMN]).
Main outcomes and measures: Nocturnal sleep variables, including total sleep time, sleep maintenance, rapid eye movement (REM) latency, and time spent in REM sleep and stages 1, 2, and 3 of non-REM (NREM1, NREM2, and NREM3, respectively) sleep, and wake after sleep onset. Neurotransmitter, tau, and total neuronal counts of LC, LHA, and TMN.
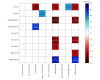
Results: Among 19 patients included in the clinicopathological correlation analysis, the mean (SD) age at death was 70.53 (7.75) years; 10 patients (52.6%) were female; and all patients were White. After adjusting for primary diagnosis, age, sex, and time between sleep analyses and death, greater numbers of LHA and TMN neurons were correlated with decreased homeostatic sleep drive, as observed by less total sleep time (LHA: r = -0.63; P = .009; TMN: r = -0.62; P = .008), lower sleep maintenance (LHA: r = -0.85; P < .001; TMN: r = -0.78; P < .001), and greater percentage of wake after sleep onset (LHA: r = 0.85; P < .001; TMN: r = 0.78; P < .001). In addition, greater numbers of LHA and TMN neurons were correlated with less NREM2 sleep (LHA: r = -0.76; P < .001; TMN: r = -0.73; P < .001). A greater number of TMN neurons was also correlated with less REM sleep (r = -0.61; P = .01). A greater number of LC neurons was mainly correlated with less total sleep time (r = -0.68; P = .008) and greater REM latency (r = 0.71; P = .006). The AD-predominant group had significantly greater sleep drive, including higher total sleep time (mean [SD], 0.49 [1.18] vs -1.09 [1.37]; P = .03), higher sleep maintenance (mean [SD], 0.18 [1.22] vs -1.53 [1.78]; P = .02), and lower percentage of wake after sleep onset during sleep period time (mean [SD], -0.18 [1.20] vs 1.49 [1.72]; P = .02) than the PSP-predominant group based on unbiased k-means clustering and principal component analyses.
Conclusions and relevance: In this cohort study, subcortical wake-promoting neurons were significantly correlated with sleep phenotypes in patients with AD and PSP, suggesting that the loss of wake-promoting neurons among patients with neurodegenerative conditions may disturb the control of sleep-wake homeostasis. These findings suggest that the subcortical system is a primary mechanism associated with sleep disturbances in the early stages of neurodegenerative diseases.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

