Efficacy and Safety of Daprodustat for Treatment of Anemia of Chronic Kidney Disease in Incident Dialysis Patients: A Randomized Clinical Trial
- PMID: 35377393
- PMCID: PMC8981070
- DOI: 10.1001/jamainternmed.2022.0605
Efficacy and Safety of Daprodustat for Treatment of Anemia of Chronic Kidney Disease in Incident Dialysis Patients: A Randomized Clinical Trial
Abstract
Importance: Daprodustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor, is being evaluated as an oral alternative to conventional erythropoiesis-stimulating agent (ESA) therapy. Few studies of anemia treatment in an incident dialysis (ID) population have been reported.
Objective: To evaluate the efficacy and safety of daprodustat vs darbepoetin alfa in treating anemia of chronic kidney disease in ID patients.
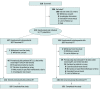
Design, setting, and participants: This prospective, randomized, open-label clinical trial was conducted from May 11, 2017, through September 24, 2020, in 90 centers across 14 countries. Patients with advanced CKD were eligible if they planned to start dialysis within 6 weeks from screening or had started and received hemodialysis (HD) or peritoneal dialysis (PD) within 90 days before randomization, had a screening hemoglobin (Hb) concentration of 8.0 to 10.5 g/dL (to convert to grams per liter, multiply by 10) and a randomization Hb of 8.0 to 11.0 g/dL, were ESA-naive or had received limited ESA treatment, and were iron-replete.
Interventions: Randomized 1:1 to daprodustat or darbepoetin alfa.
Main outcomes and measures: The primary analysis in the intent-to-treat population evaluated the mean change in Hb concentration from baseline to evaluation period (weeks 28-52) to assess noninferiority of daprodustat vs darbepoetin alfa (noninferiority margin, -0.75 g/dL). The mean monthly intravenous (IV) iron dose from baseline to week 52 was the principal secondary end point. Rates of treatment-emergent and serious adverse events (AEs) were also compared between treatment groups to assess safety and tolerability.
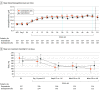
Results: A total of 312 patients (median [IQR] age, 55 [45-65] years; 194 [62%] male) were randomized to either daprodustat (157 patients; median [IQR] age, 52.0 [45-63] years; 96 [61%] male) or darbepoetin alfa (155 patients; median [IQR] age, 56.0 [45-67] years; 98 [63%] male); 306 patients (98%) completed the trial. The mean (SD) Hb concentration during the evaluation period was 10.5 (1.0) g/dL for the daprodustat and 10.6 (0.9) g/dL for the darbepoetin alfa group, with an adjusted mean treatment difference of -0.10 g/dL (95% CI, -0.34 to 0.14 g/dL), indicating noninferiority. There was a reduction in mean monthly IV iron use from baseline to week 52 in both treatment groups; however, daprodustat was not superior compared with darbepoetin alfa in reducing monthly IV iron use (adjusted mean treatment difference, 19.4 mg [95% CI, -11.0 to 49.9 mg]). Adverse event rates were 76% for daprodustat vs 72% for darbepoetin alfa.
Conclusions and relevance: This randomized clinical trial found that daprodustat was noninferior to darbepoetin alfa in treating anemia of CKD and may represent a potential oral alternative to a conventional ESA in the ID population.
Trial registration: ClinicalTrials.gov Identifier: NCT03029208.
Conflict of interest statement
Figures

References
-
- Fort J, Cuevas X, García F, et al. ; ANSWER study . Mortality in incident haemodialysis patients: time-dependent haemoglobin levels and erythropoiesis-stimulating agent dose are independent predictive factors in the ANSWER study. Nephrol Dial Transplant. 2010;25(8):2702-2710. doi:10.1093/ndt/gfq073 - DOI - PubMed

