Association of Maintenance Intravenous Immunoglobulin With Prevention of Relapse in Adult Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
- PMID: 35377395
- PMCID: PMC8981066
- DOI: 10.1001/jamaneurol.2022.0489
Association of Maintenance Intravenous Immunoglobulin With Prevention of Relapse in Adult Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
Abstract
Importance: Recent studies suggest that maintenance intravenous immunoglobulin (IVIG) may be an effective treatment to prevent relapses in myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD); however, most of these studies had pediatric cohorts, and few studies have evaluated IVIG in adult patients.
Objective: To determine the association of maintenance IVIG with the prevention of disease relapse in a large adult cohort of patients with MOGAD.
Design, setting, and participants: This was a retrospective cohort study conducted from January 1, 2010, to October 31, 2021. Patients were recruited from 14 hospitals in 9 countries and were included in the analysis if they (1) had a history of 1 or more central nervous system demyelinating attacks consistent with MOGAD, (2) had MOG-IgG seropositivity tested by cell-based assay, and (3) were age 18 years or older when starting IVIG treatment. These patients were retrospectively evaluated for a history of maintenance IVIG treatment.
Exposures: Maintenance IVIG.
Main outcomes and measures: Relapse rates while receiving maintenance IVIG compared with before initiation of therapy.
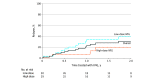
Results: Of the 876 adult patients initially identified with MOGAD, 59 (median [range] age, 36 [18-69] years; 33 women [56%]) were treated with maintenance IVIG. IVIG was initiated as first-line immunotherapy in 15 patients (25%) and as second-line therapy in 37 patients (63%) owing to failure of prior immunotherapy and in 7 patients (12%) owing to intolerance to prior immunotherapy. The median (range) annualized relapse rate before IVIG treatment was 1.4 (0-6.1), compared with a median (range) annualized relapse rate while receiving IVIG of 0 (0-3) (t108 = 7.14; P < .001). Twenty patients (34%) had at least 1 relapse while receiving IVIG with a median (range) time to first relapse of 1 (0.03-4.8) years, and 17 patients (29%) were treated with concomitant maintenance immunotherapy. Only 5 of 29 patients (17%) who received 1 g/kg of IVIG every 4 weeks or more experienced disease relapse compared with 15 of 30 patients (50%) treated with lower or less frequent dosing (hazard ratio, 3.31; 95% CI, 1.19-9.09; P = .02). At final follow-up, 52 patients (88%) were still receiving maintenance IVIG with a median (range) duration of 1.7 (0.5-9.9) years of therapy. Seven of 59 patients (12%) discontinued IVIG therapy: 4 (57%) for inefficacy, 2 (29%) for adverse effects, and 1 (14%) for a trial not receiving therapy after a period of disease inactivity.
Conclusions and relevance: Results of this retrospective, multicenter, cohort study of adult patients with MOGAD suggest that maintenance IVIG was associated with a reduction in disease relapse. Less frequent and lower dosing of IVIG may be associated with treatment failure. Future prospective randomized clinical trials are warranted to confirm these findings.
Conflict of interest statement
Figures
References
-
- Jarius S, Ruprecht K, Kleiter I, et al. ; Neuromyelitis Optica Study Group (NEMOS) . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi:10.1186/s12974-016-0718-0 - DOI - PMC - PubMed
-
- Jarius S, Ruprecht K, Kleiter I, et al. ; in cooperation with the Neuromyelitis Optica Study Group (NEMOS) . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. doi:10.1186/s12974-016-0717-1 - DOI - PMC - PubMed