The action of Con-ikot-ikot toxin on single AMPA-type glutamate receptors
- PMID: 35377397
- PMCID: PMC9195068
- DOI: 10.1085/jgp.202112912
The action of Con-ikot-ikot toxin on single AMPA-type glutamate receptors
Abstract
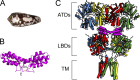
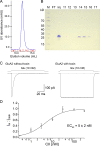
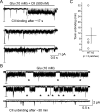
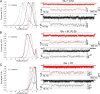
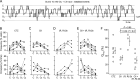
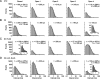
Conotoxins are a large group of naturally occurring toxic peptides produced by the predatory sea snails of the genus Conus. Many of these toxins target ion channels, often with high specificity and affinity. As such, they have proven to be invaluable for basic research, as well as acting as leads for therapeutic strategies. Con-ikot-ikot is the only conotoxin so far identified that targets AMPA-type glutamate receptors, the main mediators of excitatory neurotransmission in the vertebrate brain. Here, we describe how the toxin modifies the activity of AMPA receptors at the single-channel level. The toxin binds to the AMPA receptor with EC50 of 5 nM, and once bound takes minutes to wash out. As shown previously, it effectively blocks desensitization of AMPA receptors; however, compared to other desensitization blockers, it is a poor stabilizer of the open channel because toxin-bound AMPA receptors undergo frequent brief closures. We propose that this is a direct consequence of the toxin's unique binding mode to the ligand-binding domains (LBDs). Unlike other blockers of desensitization, which stabilize individual dimers within an AMPA receptor tetramer, the toxin immobilizes all four LBDs of the tetramer. This result further emphasizes that quaternary reorganization of independent LBD dimers is essential for the full activity of AMPA receptors.
© 2022 Baranovic et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

