Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes
- PMID: 35377461
- PMCID: PMC8978608
- DOI: 10.1002/14651858.CD003935.pub5
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes
Abstract
Background: Infants born preterm (before 37 weeks' gestation) are at risk of respiratory distress syndrome (RDS) and need for respiratory support due to lung immaturity. One course of prenatal corticosteroids, administered to women at risk of preterm birth, reduces the risk of respiratory morbidity and improves survival of their infants, but these benefits do not extend beyond seven days. Repeat doses of prenatal corticosteroids have been used for women at ongoing risk of preterm birth more than seven days after their first course of corticosteroids, with improvements in respiratory outcomes, but uncertainty remains about any long-term benefits and harms. This is an update of a review last published in 2015.
Objectives: To assess the effectiveness and safety, using the best available evidence, of a repeat dose(s) of prenatal corticosteroids, given to women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids with the primary aim of reducing fetal and neonatal mortality and morbidity.
Search methods: For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP), and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials, including cluster-randomised trials, of women who had already received one course of corticosteroids seven or more days previously and were still at risk of preterm birth, randomised to further dose(s) or no repeat doses, with or without placebo. Quasi-randomised trials were excluded. Abstracts were accepted if they met specific criteria. All trials had to meet criteria for trustworthiness, including a search of the Retraction Watch database for retractions or expressions of concern about the trials or their publications.
Data collection and analysis: We used standard Cochrane Pregnancy and Childbirth methods. Two review authors independently selected trials, extracted data, and assessed trial quality and scientific integrity. We chose primary outcomes based on clinical importance as measures of effectiveness and safety, including serious outcomes, for the women and their fetuses/infants, infants in early childhood (age two to less than five years), the infant in mid- to late childhood (age five to less than 18 years) and the infant as an adult. We assessed risk of bias at the outcome level using the RoB 2 tool and assessed certainty of evidence using GRADE.
Main results: We included 11 trials (4895 women and 5975 babies). High-certainty evidence from these trials indicated that treatment of women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids with repeat dose(s) of corticosteroids, compared with no repeat corticosteroid treatment, reduced the risk of their infants experiencing the primary infant outcome of RDS (risk ratio (RR) 0.82, 95% confidence interval (CI) 0.74 to 0.90; 3540 babies; number needed to treat for an additional beneficial outcome (NNTB) 16, 95% CI 11 to 29) and had little or no effect on chronic lung disease (RR 1.00, 95% CI 0.83 to 1.22; 5661 babies). Moderate-certainty evidence indicated that the composite of serious infant outcomes was probably reduced with repeat dose(s) of corticosteroids (RR 0.88, 95% CI 0.80 to 0.97; 9 trials, 5736 babies; NNTB 39, 95% CI 24 to 158), as was severe lung disease (RR 0.83, 95% CI 0.72 to 0.97; NNTB 45, 95% CI 27 to 256; 4955 babies). Moderate-certainty evidence could not exclude benefit or harm for fetal or neonatal or infant death less than one year of age (RR 0.95, 95% CI 0.73 to 1.24; 5849 babies), severe intraventricular haemorrhage (RR 1.13, 95% CI 0.69 to 1.86; 5066 babies) and necrotising enterocolitis (RR 0.84, 95% CI 0.59 to 1.22; 5736 babies). In women, moderate-certainty evidence found little or no effect on the likelihood of a caesarean birth (RR 1.03, 95% CI 0.98 to 1.09; 4266 mothers). Benefit or harm could not be excluded for maternal death (RR 0.32, 95% 0.01 to 7.81; 437 women) and maternal sepsis (RR 1.13, 95% CI 0.93 to 1.39; 4666 mothers). The evidence was unclear for risk of adverse effects and discontinuation of therapy due to maternal adverse effects. No trials reported breastfeeding status at hospital discharge or risk of admission to the intensive care unit. At early childhood follow-up, moderate- to high-certainty evidence identified little or no effect of exposure to repeat prenatal corticosteroids compared with no repeat corticosteroids for primary outcomes relating to neurodevelopment (neurodevelopmental impairment: RR 0.97, 95% CI 0.85 to 1.10; 3616 children), survival without neurodevelopmental impairment (RR 1.01, 95% CI 0.98 to 1.04; 3845 children) and survival without major neurodevelopmental impairment (RR 1.02, 95% CI 0.98 to 1.05; 1816 children). An increase or decrease in the risk of death since randomisation could not be excluded (RR 1.06, 95% CI 0.81 to 1.40; 5 trials, 4565 babies randomised). At mid-childhood follow-up, moderate-certainty evidence identified little or no effect of exposure to repeat prenatal corticosteroids compared with no repeat corticosteroids on survival free of neurocognitive impairment (RR 1.01, 95% CI 0.95 to 1.08; 963 children) or survival free of major neurocognitive impairment (RR 1.00, 95% CI 0.97 to 1.04; 2682 children). Benefit or harm could not be excluded for death since randomisation (RR 0.93, 95% CI 0.69 to 1.26; 2874 babies randomised) and any neurocognitive impairment (RR 0.96, 95% CI 0.72 to 1.29; 897 children). No trials reported data for follow-up into adolescence or adulthood. Risk of bias across outcomes was generally low although there were some concerns of bias. For childhood follow-up, most outcomes had some concerns of risk of bias due to missing data from loss to follow-up.
Authors' conclusions: The short-term benefits for babies included less respiratory distress and fewer serious health problems in the first few weeks after birth with repeat dose(s) of prenatal corticosteroids for women still at risk of preterm birth seven days or more after an initial course. The current available evidence reassuringly shows no significant harm for the women or child in early and mid-childhood, although no benefit. Further research is needed on the long-term benefits and risks for the baby into adulthood.
Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AW: reports working as a health professional but in an unrelated area (as an Endocrinology advanced trainee).
CM: reports previously publishing on repeat prenatal corticosteroids, being an investigator in the six‐ to eight‐year follow‐up studies of the Australasian Collaborative Trial of Repeat Doses of Corticosteroid for the Prevention of Neonatal Respiratory Disease (Crowther 2006), and working as a health professional for Counties Manukau Health.
PM: reports being an Editor for Cochrane Pregnancy and Childbirth, but was not involved in the editorial process for this review.
JH: reports giving multiple lectures and publishing review articles which relate to the material included in this review, none directly reporting the contents of the review. Investigator in the two‐year and six‐ to eight‐year follow‐up studies of the Australasian Collaborative Trial of Repeat Doses of Corticosteroid for the Prevention of Neonatal Respiratory Disease (Crowther 2006). Funding for the ACTORDS follow‐up studies was updated received from: the National Health and Medical Research Council of Australia; Channel 7 Research Foundation, South Australia; the Women's and Children's Research Foundation, Adelaide, South Australia; Discipline of Obstetrics and Gynaecology, University of Adelaide, South Australia; Health Research Council of New Zealand; Auckland Medical Research Foundation, New Zealand. None of the funding bodies had any role in the conduct of the study, analysis or decision to publish.
CAC: reports being the lead investigator for the Australasian Collaborative Trial of Repeat Doses of Corticosteroid for the Prevention of Neonatal Respiratory Disease and subsequent follow‐up studies (Crowther 2006).
Figures





















































































































































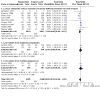









































































Update of
-
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes.Cochrane Database Syst Rev. 2015 Jul 5;2015(7):CD003935. doi: 10.1002/14651858.CD003935.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Apr 4;4:CD003935. doi: 10.1002/14651858.CD003935.pub5. PMID: 26142898 Free PMC article. Updated.
References
References to studies included in this review
Aghajafari 2002 {published data only}
-
- Aghajafari F, Murphy K, Ohlsson A, Amankwah K, Matthews S, Hannah M.Multiple versus single courses of antenatal corticosteroids for preterm birth: a pilot study. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(4):321-9. - PubMed
Crowther 2006 {published data only}
-
- ACTRN12606000318583.Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial [A multicentred randomised controlled trial of repeat doses of prenatal corticosteroid given to women who remain at risk of preterm delivery for the prevention of neonatal morbidity]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12606000318583 (first received 5 March 2001). [CENTRAL: CN-01861799]
-
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DK, Hiller JE, et al.Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861-7. - PubMed
-
- Battin M, Bevan C, Harding J.Growth in the neonatal period after repeat courses of antenatal corticosteroids: data from the ACTORDS randomised trial. Archives of Disease in Childhood Fetal & Neonatal Edition 2012;97(2):F99-105. - PubMed
-
- Battin M, Bevan C, Harding J.Repeat doses of antenatal steroids and hypothalamic-pituitary-adrenal axis (HPA) function. American Journal of Obstetrics and Gynecology 2007;197:40.e1-40.e6. - PubMed
-
- Battin MR, Bevan C, Morton SM, Harding JE.Repeat courses of antenatal corticosteroids do not alter hypothalamic-pituitary-adrenal axis function after birth; results of a randomised controlled trial. In: Pediatric Academic Societies Annual Meeting; 2004 May 1-4; San Francisco, USA. 2004.
Garite 2009 {published data only}
-
- Garite T, Kurtzman J, Maurel K, Clark R, for the Obstetrix Collaborative Research Network.Impact of a 'rescue course' of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. American Journal of Obstetrics and Gynecology 2009;200:248.e1-9. - PubMed
-
- Kurtzman J, Garite T, Clark R, Maurel K, The Obstetrix Collaborative Research Network.Impact of a "rescue course" of antenatal corticosteroids (ACS): a multicenter randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. - PubMed
-
- NCT00201643.A randomized trial comparing the impact of one versus two courses of ACS on neonatal outcome. clinicaltrials.gov/ct2/show/NCT00201643 (first received 20 September 2005).
Guinn 2001 {published data only}
-
- Guinn D, Atkinson M, Sullivan L, Lee M, MacGregor S, Parilla B, et al.Single versus weekly courses of antenatal corticosteroids for women at risk of preterm delivery: a randomized controlled trial. Obstetrics & Gynecology 2003;101(1):195.
-
- Guinn D, BMZ Study Group.Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS). American Journal of Obstetrics and Gynecology 2001;184(1):S6.
-
- Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla B, et al.Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery. JAMA 2001;286(13):1581-7. - PubMed
-
- Guinn DA, BMZ Study Group.Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS): interim analysis. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S12.
-
- Lee M, Davies J, Atkinson MW, Guinn D, BMZ Study Group.Efficacy of weekly courses of antenatal corticosteroids (ACS) in preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology 2001;184(1):S8. - PubMed
Mazumder 2008 {published data only}
-
- Mazumder P, Dutta S, Kaur J, Narang A.Single versus multiple courses of antenatal betamethasone and neonatal outcome: a randomized controlled trial. Indian Pediatrics 2008;45(8):661-7. [PMID: ] - PubMed
McEvoy 2002 {published data only}
-
- McEvoy C, Bowling S, Williamson K, Lozano D, Tolaymat L, Collins J, et al.Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. In: Pediatric Academic Societies Annual Meeting; 2001 Apr 28-May 1; Baltimore Convention Centre, Baltimore (MD). 2001:2228.
-
- McEvoy C, Bowling S, Williamson R, Lozano D, Tolaymat L, Izquierdo L, et al.The effect of a single remote course versus weekly courses of antenatal corticosteroids on functional residual capacity in preterm infants: a randomized trial. Pediatrics 2002;110:280-4. - PubMed
-
- McEvoy C.Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. Pediatric Research 2001;49(4):388A. - PubMed
McEvoy 2010 {published data only}
-
- Jordan BK, Schilling D, McEvoy CT.The longitudinal impact of rescue antenatal steroids on neonatal respiratory compliance through hospital discharge: follow up of a randomized controlled trial (RCT). In: Pediatric Academic Societies Annual Meeting; 2016 Apr 30-May 3; Baltimore (MD). 2016:3828.264. [CENTRAL: CN-02011933]
-
- McEvoy C, Schilling D, Clay N, Spitale P, Durand M.Neurodevelopmental outcome and growth in infants randomized to a single rescue course of antenatal steroids. In: Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 Apr 30-May 3; Denver (CO). 2011:3829.270.
Murphy 2008 {published and unpublished data}
-
- Asztalos E, Murphy K, Hannah M, Willan A, MACS Collaborative Group.Outcomes of children at two years after multiple courses of antenatal corticosteroids for threatened preterm birth: the Multiple Antenatal Corticosteroids study (MACS). Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1-4; Vancouver, Canada.
-
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al.Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy and Childbirth 2014;14(1):272. - PMC - PubMed
-
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al.Multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5): association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years of age (MACS-5). American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S321-2.
-
- Asztalos EV, Murphy KE, Hannah ME, Willan AR, Matthews SG, Ohlsson A, et al.Multiple courses of antenatal corticosteroids for preterm birth study: 2-year outcomes. Pediatrics 2010;126:e1045-55. - PubMed
-
- Asztalos EV, Murphy KE, Willan AR, Matthews SG, Ohlsson A, Saigal S, et al, for the MACS-5 Collaborative Group.Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). Journal of the American Medical Association Pediatrics 2013;167(12):1102-10. - PubMed
Peltoniemi 2007 {published data only}
-
- Janer C, Helve O, Pitkänen OM, Kari MA, Peltoniemi OM, Hallman M, et al.Expression of airway epithelial sodium channel in the preterm infant is related to respiratory distress syndrome but unaffected by repeat antenatal betamethasone. Neonatology 2010;97(2):132-8. - PubMed
-
- Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M.Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatrica 2007;96(5):664-8. - PubMed
-
- NCT00295464.Antenatal rescue course of glucocorticoids in threatened premature birth [Randomized trial on efficacy and safety of the antenatal rescue course of glucocorticoids in threatened premature birth (ACG Trial)]. clinicaltrials.gov/show/NCT00295464 (first received 21 February 2006).
-
- Peltoniemi O, Kari M, Lano A, Yliherva A, Puosi R, Lehtonen L, et al.Two-year follow-up of a randomized trial with repeated antenatal betamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(6):F402-6. - PubMed
-
- Peltoniemi OM, Kari MA, Halmesmaki E, Raudaskoski T, Tammela O, Uotila J, et al.The effect of second course of antenatal betamethasone (BM) on neonatal morbidity in preterm infants: a randomised trial. Pediatric Research 2005;58(2):404.
TEAMS 1999 {unpublished data only}ISRCTN46614711
-
- ISRCTN46614711.Trial of the effects of antenatal multiple courses of steroids versus a single course (TEAMS): pilot study. www.isrctn.com/ISRCTN46614711 (first received 1 March 2001).
Wapner 2006 {published data only}
-
- Borowski KS, Clark EA, Lai Y, Wapner RJ, Sorokin Y, Peaceman AM, et al.Neonatal genetic variation in steroid metabolism and key respiratory function genes and perinatal outcomes in single and multiple courses of corticosteroids. American Journal of Perinatology 2015;32(12):1126-32. [CENTRAL: CN-01169539] [PMID: ] - PMC - PubMed
-
- Church M.Auditory brainstem responses (ABRs) in neonates exposed to repeated courses of antenatal corticosteroids. In: Program of the Twenty Eighth Annual Midwinter Research Meeting of the Association for Research in Otolaryngology; 2005 Feb 19-24; New Orleans (LA). 2005:669.
-
- Dude C, Dude A, Gilner J, Swamy G, Grotegut C.Predicting preterm delivery: using the MFMU BEARS trial data to optimize corticosteroid use in women at risk for preterm delivery. Reproductive Sciences (Thousand Oaks, Calif.) 2016;23(Suppl 1):188A-9A. [CENTRAL: CN-02011932]
References to studies excluded from this review
Bontis 2011 {published data only}
-
- Bontis N, Vavilis D, Tsolakidis D, Goulis DG, Tzevelekis P, Kellartzis D, et al.Comparison of single versus multiple courses of antenatal betamethasone in patients with threatened preterm labor. Clinical and Experimental Obstetrics and Gynecology 2011;38(2):165-7. - PubMed
CTRI/2017/04/008326 {published data only}
-
- CTRI/2017/04/008326.Improving newborn survival in preterm birth [A multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries to improve newborn outcomes – Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns (ACTION-I TRIAL)]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/04/008326 (first received 10 April 2017).
CTRI/2017/05/008721 {published data only}
-
- CTRI/2017/05/008721.Improving newborn survival in late preterm birth [A multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial of antenatal corticosteroids for women at risk of imminent birth in the late preterm period in hospitals in low-resource countries to improve newborn outcomes – Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns (ACTION-II TRIAL)]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/05/008721 (first received 31 May 2017). [CENTRAL: CN-01884324]
Ernawati 2016 {published data only}
-
- Ernawati, Gumilar E, Kuntoro, Soeroso J, Dekker G.Expectant management of preterm preeclampsia in Indonesia and the role of steroids. Journal of Maternal-fetal & Neonatal Medicine 2016;29(11):1736-40. [CENTRAL: CN-01141034] [EMBASE: 2015249052] [PMID: ] - PubMed
EUCTR2009‐010759‐29‐BE {published data only}
-
- EUCTR2009-010759-29-BE.Etude clinique sur l'administration de 3 doses de betamethasone (12mg) a 18 heures d'intervalle lors d'une menace d'accouchement premature dans les grossesses gemellaires traitees par Atosiban, plutot que 2 doses de betamethasone (12mg) a 24 heures d'intervalle; afin de diminuer le risque de syndrome de detresse respiratoire chez les nouveau-nes – CORTGEM. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-010759-29-BE (first received 30 March 2009).
Gyamfi‐Bannerman 2016 {published data only}
-
- Viteri OA, Doty MS, Alrais MA, Sibai BM.471: intended administration of antenatal late preterm steroids: is a single dose enough? American Journal of Obstetrics and Gynecology 2019;220(1):S315. [CENTRAL: CN-01710606] [EMBASE: 2001405752]
IRCT2014090912789N6 {published data only}
-
- IRCT2014090912789N6.Impact of betamethasone on preterm infants outcomes [Comparing the impact of one versus two doses of betamethasone on preterm infants outcomes: a clinical trial study]. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014090912789N6 (first received 20 October 2014). [CENTRAL: CN-01862384]
IRCT2015120415634N2 {published data only}
-
- IRCT2015120415634N2.The effect of antenatal intramuscular administration of two doses of betamethasone every 12 hours on acute pulmonary complications in preterm infants born [Comparing of antenatal intramuscular administration of two doses of betamethasone every 12 hours with standard method every 24 hours on acute neonatal pulmonary complications in preterm infants born from women with preterm premature of membrane]. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015120415634N2 (first received 27 September 2017). [CENTRAL: CN-01886227]
IRCT20191202045571N1 {published data only}
-
- IRCT20191202045571N1.The effect of betamethasone injection on maternal and neonatal outcomes in pregnant women with gestational age 34–37 weeks exposed to preterm labor in Sayyad Shirazi Hospital, Gorgan. www.irct.ir/trial/44053 (first received 17 July 2020). [CENTRAL: CN-02148336]
Kashanian 2018 {published data only}
-
- Kashanian M, Eshraghi N, Sheikhansari N, Bordbar A, Khatami E.Comparison between two doses of betamethasone administration with 12 hours vs. 24 hours intervals on prevention of respiratory distress syndrome: a randomised trial. Journal of Obstetrics and Gynaecology 2018;38(6):770-6. [CENTRAL: CN-01665275] [EMBASE: 621208178] [PMID: ] - PubMed
Mercer 2001 {published data only}
-
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J.Steroids reduce fetal growth: analysis of a prospective trial. American Journal of Obstetrics and Gynecology 2001;184(1):S7.
-
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J.Weekly antenatal steroids trial in women at risk of preterm birth: a randomized trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6.
-
- Sawady J, Mercer B.Impact of repeated doses of antenatal corticosteroids on placental growth and histology. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S73.
NCT03446937 {published data only}
-
- NCT03446937.Effect of antenatal corticosteroids on neonatal morbidity. clinicaltrials.gov/show/NCT03446937 (first received 5 February 2018). [CENTRAL: CN-01483469]
Romejko‐Wolniewicz 2013 {published data only}
-
- Romejko-Wolniewicz E, Oleszczuk L, Zareba-Szczudlik J, Czajkowski K.Dosage regimen of antenatal steroids prior to preterm delivery and effects on maternal and neonatal outcomes. Journal of Maternal-Fetal and Neonatal Medicine 2013;26(3):237-41. - PubMed
Schmitz 2019 {published data only}
-
- Schmitz T, Alberti C, Ursino M, Baud O, Aupiais C.Full versus half dose of antenatal betamethasone to prevent severe neonatal respiratory distress syndrome associated with preterm birth: study protocol for a randomised, multicenter, double blind, placebo-controlled, non-inferiority trial (BETADOSE). BMC Pregnancy and Childbirth 2019;19(1):67. [CENTRAL: CN-01787973] [EMBASE: 626359019] [PMID: ] - PMC - PubMed
Sohrabvand 2001 {published data only}
-
- Sohrabvand F, Behbahani B, Kazeminejad A.Effects of single versus multiple courses of corticosteroid therapy on pregnancy results in women with PPROM. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):528.
Thorp 2000 {published data only}
-
- Thorp JA, Yeast JD, Cohen GR, Wickstrom EA, D'Angelo LJ.Repeated antenatal betamethasone and perinatal outcome. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S21.
References to studies awaiting assessment
Atarod 2014 {published data only}
-
- Atarod Z, Taghipour M, Roohanizadeh H, Fadavi S, Taghavipour M.Effects of single course and multicourse betamethasone prior to birth in the prognosis of the preterm neonates: a randomized, double-blind placebo-control clinical trial study. Journal of Research in Medical Sciences 2014;19(8):715-9. - PMC - PubMed
-
- Atarod Z.Single versus multiple courses of antenatal betamethasone: evaluation of preterm infant's outcome. www.irct.ir/trial/1175 (first received 16 May 2009).
References to ongoing studies
NCT02469519 {published data only}
-
- NCT02469519.Impact of a booster course of antenatal steroids on neonatal outcome in patients with premature rupture of the membranes [A randomized double-blinded trial comparing the impact of one versus two courses of antenatal steroids on neonatal outcome in the patient with prelabor premature rupture of the membranes]. clinicaltrials.gov/show/NCT02469519 (first received 5 June 2015). [CENTRAL: CN-01552815]
NCT02939742 {published data only}
-
- NCT02939742.Does a rescue course of betamethasone in pregnant women with PPROM decrease neonatal morbidity? [Does a repeat course of antenatal corticosteroids in pregnant women with preterm premature rupture of membranes decrease neonatal morbidity?]. clinicaltrials.gov/show/nct02939742 (first received 23 September 2016). [CENTRAL: CN-01583154]
Additional references
ACOG 2017
-
- American College of Obstetrics and Gynecology (ACOG) Committee on Obstetric Practice.Committee opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstetrics and Gynecology 2017;130(2):e102-9. [DOI: ] - PubMed
AIHW 2020
-
- Australian Institute of Health and Welfare.Australia's Mothers and Babies 2018: in Brief. Perinatal Statistics Series no. 36. Canberra (Australia): Australian Institute of Health and Welfare, 2020. [ISBN: 978-1-76054-681-6]
Antenatal Corticosteroids CPG Panel 2015
-
- Antenatal Corticosteroids Clinical Practice Guidelines Panel.Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines. Auckland (New Zealand): Liggins Institute, The University of Auckland, 2015.
Ashwood 2006
-
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DJ, Hiller JE, et al.Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861-7. - PubMed
Benediktsson 1993
-
- Benediktsson R, Lindsay RS, Noble J, Secki JR, Edwards CR.Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341:339-41. - PubMed
Blencowe 2013
Carlisle 2017
-
- Carlisle JB.Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72(8):944-52. [DOI: ] - PubMed
Cheong 2017
-
- Cheong JL, Anderson PJ, Burnett AC, Roberts G, Davis N, Hickey L, et al for the Victorian Infant Collaborative Study Group.Changing neurodevelopment at 8 Years in children born extremely preterm since the 1990s. Pediatrics 2017;139(6):e20164086. [DOI: ] - PubMed
Crowther 2019
Dalziel 2005a
-
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al.Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005;365(9474):1856-62. - PubMed
Dalziel 2005b
Dunlop 1997
-
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP.Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. Journal of Maternal-Fetal Medicine 1997;6:309-13. - PubMed
Eldridge 2021
-
- Eldridge S, Campbell MK, Campbell MJ, Drahota AK, Giraudeau B, Reeves BC, et al.Revised Cochrane risk of bias tool for randomized trials (RoB 2). Additional considerations for cluster-randomized trials (RoB 2 CRT). www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-tr... (accessed prior to 11 March 2021).
Esplin 2000
-
- Esplin M, Fausett M, Smith S, Oshiro B, Porter TF, Branch DW, et al.Multiple courses of antenatal steroids associated with a delay in long-term psychomotor development in children with birth weight < 1500 grams. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S24.
Fowden 1996
-
- Fowden AL, Szemere J, Hughes RS, Forhead AJ.The effects of cortisol on the growth rate of the sheep fetus during late gestation. Journal of Endocrinology 1996;151:97-105. - PubMed
French 1998
-
- French N, Hagan R, Evans S, Godfrey M, Newnham J.Repeated antenatal corticosteroids: behaviour outcomes in a regional population of very preterm infants. Pediatric Research 1998;43:214A.
French 1999
-
- French N, Hagan R, Evans S, Godfrey M, Newnham J.Repeated antenatal corticosteroids: size at birth and subsequent development. American Journal of Obstetrics and Gynecology 1999;180:114-21. - PubMed
French 2004
-
- French N, Hagan R, Evans S, Mullan A, Newnham J.Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behaviour. American Journal of Obstetrics and Gynecology 2004;190:588-95. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 20 September 2021. Available at gradepro.org.
Hasbargen 2001
-
- Hasbargen U, Reber D, Versmold H, Schulze A.Growth and development of children to 4 years of age after repeated antenatal steroid administration. European Journal of Pediatrics 2001;160:552-5. - PubMed
Higgins 2019
-
- Higgins JP, Savovic J, Page MJ, Sterne JA.Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [ ]. www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed prior to 11 March 2022).
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Ikegami 1997
-
- Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P.Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. American Journal of Respiratory and Critical Care Medicine 1997;156:178-84. - PubMed
Jensen 2002
-
- Jensen EC, Gallaher BW, Breier BH, Harding JE.The effect of a chronic maternal cortisol infusion on the late gestation fetal sheep. Journal of Endocrinology 2002;174:27-36. - PubMed
Jobe 1994
-
- Jobe AH.National Institutes of Health. Report of the Consensus Development Conference on the Effect of Corticosteroids for Fetal Lung Maturation on Perinatal Outcomes. Bethesda (MD): US Department of Health and Human Services, Public Health Service, 1994.
Liggins 1972
-
- Liggins GC, Howie RN.A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515-25. - PubMed
McGoldrick 2020
McKenna 2000
-
- McKenna DS, Witter GM, Nagaraja HN, Samuels P.The effects of repeat doses of antenatal corticosteroids on maternal adrenal function. American Journal of Obstetrics and Gynecology 2000;183:669-73. - PubMed
McLaughlin 2003
-
- McLaughlin KJ, Crowther CA, Walker N, Harding JE.Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:101-6. - PubMed
Mildenhall 2006
Moise 1995
-
- Moise AA, Wearden ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN.Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics 1995;95:845-50. - PubMed
Newnham 1999
-
- Newnham JP, Evans S, Godfrey M, Huang W, Ikegami M, Jobe A.Maternal, but not fetal, administration of corticosteroids restricts fetal growth. Journal of Maternal-Fetal Medicine 1999;8(3):81-7. - PubMed
NIH 1995
-
- NIH Consensus Development Panel.Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995;273:413-8. - PubMed
Padbury 1996
-
- Padbury JF, Ervin G, Polk D.Extrapulmonary effects of antenatally administered steroids. Journal of Pediatrics 1996;128:167-72. - PubMed
RevMan Web 2021 [Computer program]
-
- Review Manager Web (RevMan Web).Version 3.9.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Roberts 2006
Rojas‐Reyes 2012
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).GRADE handbook for grading quality of evidence and strength of recommendations (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seckl 2004
-
- Seckl JR, Meaney MJ.Glucocorticoid programming. Annals of the New York Academy of Sciences 2004;1032:63-84. - PubMed
Thorp 2002
-
- Thorp JA, Etzenhouser J, O'Connor M, Jones A, Jones P, Belden B, et al.Effects of phenobarbital and multiple-dose antenatal/postnatal steroid on developmental outcome at age 7 years. American Journal of Obstetrics and Gynecology 2002;185(6):S87.
Tschanz 1995
-
- Tschanz SA, Danke BM, Burri PH.Influence of postnatally administered glucocorticoids on rat lung growth. Biology of the Neonate 1995;68:229-45. - PubMed
Utama 2018
Wan 2014
WHO 2015
-
- World Health Organization.WHO recommendations on interventions to improve preterm birth outcomes: evidence base. www.who.int/reproductivehealth/publications/maternal_perinatal_health/pr... (accessed prior to 11 March 2022). - PubMed
References to other published versions of this review
Crowther 2000
Crowther 2007
Crowther 2011
Crowther 2015
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

