Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results From the EMPULSE Trial
- PMID: 35377706
- PMCID: PMC9311476
- DOI: 10.1161/CIRCULATIONAHA.122.059725
Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results From the EMPULSE Trial
Abstract
Background: Patients hospitalized for acute heart failure experience poor health status, including a high burden of symptoms and physical limitations, and poor quality of life. SGLT2 (sodium-glucose cotransporter 2) inhibitors improve health status in chronic heart failure, but their effect on these outcomes in acute heart failure is not well characterized. We investigated the effects of the SGLT2 inhibitor empagliflozin on symptoms, physical limitations, and quality of life, using the Kansas City Cardiomyopathy Questionnaire (KCCQ) in the EMPULSE trial (Empagliflozin in Patients Hospitalized With Acute Heart Failure Who Have Been Stabilized).
Methods: Patients hospitalized for acute heart failure were randomized to empagliflozin 10 mg daily or placebo for 90 days. The KCCQ was assessed at randomization and 15, 30, and 90 days. The effects of empagliflozin on the primary end point of clinical benefit (hierarchical composite of all-cause death, heart failure events, and a 5-point or greater difference in KCCQ Total Symptom Score [TSS] change from baseline to 90 days) were examined post hoc across the tertiles of baseline KCCQ-TSS. In prespecified analyses, changes (randomization to day 90) in KCCQ domains, including TSS, physical limitations, quality of life, clinical summary, and overall summary scores were evaluated using a repeated measures model.
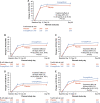
Results: In total, 530 patients were randomized (265 each arm). Baseline KCCQ-TSS was low overall (mean [SD], 40.8 [24.0] points). Empagliflozin-treated patients experienced greater clinical benefit across the range of KCCQ-TSS, with no treatment effect heterogeneity (win ratio [95% CIs] from lowest to highest tertile: 1.49 [1.01-2.20], 1.37 [0.94-1.99], and 1.48 [1.00-2.20], respectively; P for interaction=0.94). Beneficial effects of empagliflozin on health status were observed as early as 15 days and persisted through 90 days, at which point empagliflozin-treated patients experienced a greater improvement in KCCQ TSS, physical limitations, quality of life, clinical summary, and overall summary (placebo-adjusted mean differences [95% CI]: 4.45 [95% CI, 0.32-8.59], P=0.03; 4.80 [95% CI, 0.00-9.61], P=0.05; 4.66 [95% CI, 0.32-9.01], P=0.04; 4.85 [95% CI, 0.77-8.92], P=0.02; and 4.40 points [95% CI, 0.33-8.48], P=0.03, respectively).
Conclusions: Initiation of empagliflozin in patients hospitalized for acute heart failure produced clinical benefit regardless of the degree of symptomatic impairment at baseline, and improved symptoms, physical limitations, and quality of life, with benefits seen as early as 15 days and maintained through 90 days.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT0415775.
Trial registration: ClinicalTrials.gov NCT04157751.
Keywords: SGLT2 inhibitor; acute heart failure; quality of life.
Figures


Comment in
-
SGLT2 Inhibitors: From Antihyperglycemic Agents to All-Around Heart Failure Therapy.Circulation. 2022 Jul 26;146(4):299-302. doi: 10.1161/CIRCULATIONAHA.122.060348. Epub 2022 Jul 25. Circulation. 2022. PMID: 35877834 No abstract available.
References
-
- Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, Tay WT, Dickstein K, Ertl G, Hassanein M, et al.. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. 2020;8:e411–e422. doi: 10.1016/S2214-109X(20)30004-8 - PubMed
-
- Belkin M, Wussler D, Gualandro DM, Shrestha S, Strebel I, Goudev A, Maeder MT, Walter J, Flores D, Kozhuharov N, et al.. Effect of a strategy of comprehensive vasodilation versus usual care on health-related quality of life among patients with acute heart failure. ESC Heart Fail. 2021;8:4218–4227. doi: 10.1002/ehf2.13543 - PMC - PubMed
-
- Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al.. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929 - PubMed
-
- Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, et al.. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138 - PMC - PubMed

