Screening for Inhibitors of YAP Nuclear Localization Identifies Aurora Kinase A as a Modulator of Lung Fibrosis
- PMID: 35377835
- PMCID: PMC9798384
- DOI: 10.1165/rcmb.2021-0428OC
Screening for Inhibitors of YAP Nuclear Localization Identifies Aurora Kinase A as a Modulator of Lung Fibrosis
Abstract
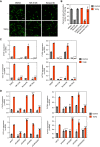
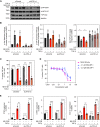
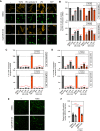
Idiopathic pulmonary fibrosis is a progressive lung disease with limited therapeutic options that is characterized by pathological fibroblast activation and aberrant lung remodeling with scar formation. YAP (Yes-associated protein) is a transcriptional coactivator that mediates mechanical and biochemical signals controlling fibroblast activation. We previously identified HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors (statins) as YAP inhibitors based on a high-throughput small-molecule screen in primary human lung fibroblasts. Here we report that several Aurora kinase inhibitors were also identified from the top hits of this screen. MK-5108, a highly selective inhibitor for AURKA (Aurora kinase A), induced YAP phosphorylation and cytoplasmic retention and significantly reduced profibrotic gene expression in human lung fibroblasts. The inhibitory effect on YAP nuclear translocation and profibrotic gene expression is specific to inhibition of AURKA, but not Aurora kinase B or C, and is independent of the Hippo pathway kinases LATS1 and LATS2 (Large Tumor Suppressor 1 and 2). Further characterization of the effects of MK-5108 demonstrate that it inhibits YAP nuclear localization indirectly via effects on actin polymerization and TGFβ (Transforming Growth Factor β) signaling. In addition, MK-5108 treatment reduced lung collagen deposition in the bleomycin mouse model of pulmonary fibrosis. Our results reveal a novel role for AURKA in YAP-mediated profibrotic activity in fibroblasts and highlight the potential of small-molecule screens for YAP inhibitors for identification of novel agents with antifibrotic activity.
Keywords: Aurora kinase A inhibitor; YAP; drug screening; human lung fibroblast; idiopathic pulmonary fibrosis.
Figures




Comment in
-
A Glowing Opportunity to Target YAP in Lung Fibrosis.Am J Respir Cell Mol Biol. 2022 Jul;67(1):1-2. doi: 10.1165/rcmb.2022-0082ED. Am J Respir Cell Mol Biol. 2022. PMID: 35503038 Free PMC article. No abstract available.
References
-
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association An official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline Am J Respir Crit Care Med 2015192e3–e19.. [Published erratum appears in Am J Respir Crit Care Med 192:644.]
-
- Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med . 2018;378:1811–1823. - PubMed
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083–2092. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2071–2082. - PubMed
-
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet . 2017;389:1941–1952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

