Association of Transthyretin Val122Ile Variant With Incident Heart Failure Among Black Individuals
- PMID: 35377943
- PMCID: PMC8981072
- DOI: 10.1001/jama.2022.2896
Association of Transthyretin Val122Ile Variant With Incident Heart Failure Among Black Individuals
Erratum in
-
Incorrect Spelling of Author Name.JAMA. 2022 May 10;327(18):1825. doi: 10.1001/jama.2022.6725. JAMA. 2022. PMID: 35536276 Free PMC article. No abstract available.
Abstract
Importance: A genetic variant in the TTR gene (rs76992529; Val122Ile), present more commonly in individuals with African ancestry (population frequency: 3%-4%), causes misfolding of the tetrameric transthyretin protein complex that accumulates as extracellular amyloid fibrils and results in hereditary transthyretin amyloidosis.
Objective: To estimate the association of the amyloidogenic Val122Ile TTR variant with the risk of heart failure and mortality in a large, geographically diverse cohort of Black individuals.
Design, setting, and participants: Retrospective population-based cohort study of 7514 self-identified Black individuals living in the US participating in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study with genetic data available and without heart failure at baseline. The participants were enrolled at the baseline visit (2003-2007). The end of follow-up for the majority of outcomes was on December 31, 2018. All-cause mortality data were available through December 31, 2020.
Exposures: TTR Val122Ile (rs76992529) genotype.
Main outcome and measures: The primary outcome was incident heart failure (first hospitalization for heart failure or death due to heart failure). The secondary outcomes were heart failure mortality, cardiovascular mortality, and all-cause mortality. The multivariable Cox proportional hazards regression analyses were adjusted for genetic ancestry and demographic, clinical, and social factors.
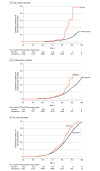
Results: Among 7514 Black participants (median age, 64 years [IQR, 57-70 years]; 61% women), the population frequency of the TTR Val122Ile variant was 3.1% (232 variant carriers and 7282 noncarriers). During a median follow-up of 11.1 years (IQR, 5.9-13.5 years), incident heart failure occurred in 535 individuals (34 variant carriers and 501 noncarriers) and the incidence of heart failure was 15.64 per 1000 person-years among variant carriers vs 7.16 per 1000 person-years among noncarriers (adjusted hazard ratio [HR], 2.43 [95% CI, 1.71-3.46]; P < .001). Deaths due to heart failure occurred in 141 individuals (13 variant carriers and 128 noncarriers) and the incidence of heart failure mortality was 6.11 per 1000 person-years among variant carriers vs 1.85 per 1000 person-years among noncarriers (adjusted HR, 4.19 [95% CI, 2.33-7.54]; P < .001). Deaths due to cardiovascular causes occurred in 793 individuals (34 variant carriers and 759 noncarriers) and the incidence of cardiovascular death was 15.18 per 1000 person-years among variant carriers vs 10.61 per 1000 person-years among noncarriers (adjusted HR, 1.69 [95% CI, 1.19-2.39]; P = .003). Deaths due to any cause occurred in 2715 individuals (100 variant carriers and 2615 noncarriers) and the incidence of all-cause mortality was 41.46 per 1000 person-years among variant carriers vs 33.94 per 1000 person-years among noncarriers (adjusted HR, 1.46 [95% CI, 1.19-1.78]; P < .001). There was no significant interaction between TTR variant carrier status and sex on incident heart failure and the secondary outcomes.
Conclusions and relevance: Among a cohort of Black individuals living in the US, being a carrier of the TTR Val122Ile variant was significantly associated with an increased risk of heart failure.
Conflict of interest statement
Figures



Comment in
-
Heart Failure, Precision Medicine, and Incremental Equity: The Case of Hereditary Amyloid Cardiomyopathy.JAMA. 2022 Apr 12;327(14):1341-1343. doi: 10.1001/jama.2022.2360. JAMA. 2022. PMID: 35377944 Free PMC article. No abstract available.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

