UK clinical guideline for the prevention and treatment of osteoporosis
- PMID: 35378630
- PMCID: PMC8979902
- DOI: 10.1007/s11657-022-01061-5
UK clinical guideline for the prevention and treatment of osteoporosis
Erratum in
-
Correction: UK clinical guideline for the prevention and treatment of osteoporosis.Arch Osteoporos. 2022 May 19;17(1):80. doi: 10.1007/s11657-022-01115-8. Arch Osteoporos. 2022. PMID: 35585444 Free PMC article. No abstract available.
Abstract
The National Osteoporosis Guideline Group (NOGG) has revised the UK guideline for the assessment and management of osteoporosis and the prevention of fragility fractures in postmenopausal women, and men age 50 years and older. Accredited by NICE, this guideline is relevant for all healthcare professionals involved in osteoporosis management.
Introduction: The UK National Osteoporosis Guideline Group (NOGG) first produced a guideline on the prevention and treatment of osteoporosis in 2008, with updates in 2013 and 2017. This paper presents a major update of the guideline, the scope of which is to review the assessment and management of osteoporosis and the prevention of fragility fractures in postmenopausal women, and men age 50 years and older.
Methods: Where available, systematic reviews, meta-analyses and randomised controlled trials were used to provide the evidence base. Conclusions and recommendations were systematically graded according to the strength of the available evidence.
Results: Review of the evidence and recommendations are provided for the diagnosis of osteoporosis, fracture-risk assessment and intervention thresholds, management of vertebral fractures, non-pharmacological and pharmacological treatments, including duration and monitoring of anti-resorptive therapy, glucocorticoid-induced osteoporosis, and models of care for fracture prevention. Recommendations are made for training; service leads and commissioners of healthcare; and for review criteria for audit and quality improvement.
Conclusion: The guideline, which has received accreditation from the National Institute of Health and Care Excellence (NICE), provides a comprehensive overview of the assessment and management of osteoporosis for all healthcare professionals involved in its management. This position paper has been endorsed by the International Osteoporosis Foundation and by the European Society for the Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases.
Keywords: Fracture; Guideline; NOGG; Osteoporosis.
© 2022. The Author(s).
Conflict of interest statement
The National Osteoporosis Guideline Group (NOGG) Guideline Writing Group is divided into two groups, a Guideline Development Group (GDG) and an Expert Advisory Group (EAG). The GDG consists of 8 voting members (CLG, JC, JE, JK, RL, SL, ZP, JT), and 3 lay members (JB, JP, NV). One member has a financial disclosure, unrelated to drug development (JC received honoraria for speaking at a CME approved bone academy meeting and for consultancy relating to the characterisation of people at very high fracture risk), the remaining 10 have no financial disclosures. ZP is funded by the National Institute for Health Research (NIHR) (Clinician Scientist Award (CS-2018–18-ST2-010)/NIHR Academy). ZP has undertaken consultancy for non-promotional activities for UCB and waived any rights to any fees. One member (JK) is also a member of the FRAX group, hence all FRAX-based recommendations had to be agreed by consensus of all the GDG. The Chair (CLG) has no disclosures. The EAG is composed of 9 members, all of whom have relevant conflicts of interest. DA has received advisory/speaking fees from UCB and Internis Pharma and has shares in GSK. CC has received advisory/speaking fees from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda, and UCB. NG is on the advisory board of UCB, Novo Nordisk, Shire/Takeda and has received fees from UCB and Takeda. NCH has received advisory board/consultancy fees/honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, UCB, Radius, Servier, Shire, Consilient Healthcare, and Internis Pharma. EMC has received advisory/honoraria from Amgen, Consilient Healthcare, Kyowa Kirin, Eli Lilly, Fresenius Kabi, Internis, ObsEva, Radius Inc and Redx Pharma and received research funding from Amgen, Internis, and Radius Inc. KM has received conference sponsorship from Abbvie, UCB, Novartis, Kyowa Kirin, Lilly, Pfizer, and Internis, and advisory/honoraria from Alexion. KP received advisory/speaker fees from UCB which were then donated to charities. DR has received advisory fees from UCB and Osteolabs. MS has received advisory/speaker fees from UCB, and speaker fees from Amgen and Gedeon Richter. All members of the GDG and EAG have read NICE’s ‘Policy on declaring and managing interests for NICE advisory boards’ (version 1.1, July 2019). Disclosures of all members of both groups are available on the NOGG website. Both the GDG and the EAG attend online meetings and take part in email discussions. However, voting on the recommendations is restricted to the GDG. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Figures

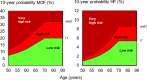
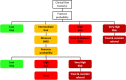

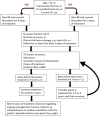
Comment in
-
Letter to Editor: Our concerns about HRT not having a priority as a treatment for osteoporosis in the NOGG guidelines.Osteoporos Int. 2023 Apr;34(4):815-816. doi: 10.1007/s00198-022-06619-0. Epub 2022 Dec 28. Osteoporos Int. 2023. PMID: 36575344 No abstract available.
References
-
- Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M. Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009;62:105–108. doi: 10.1016/j.maturitas.2008.11.022. - DOI - PubMed
-
- Söreskog E, Lindberg I, Kanis JA, Åkesson KE, Willems D, Lorentzon M, Ström O, Berling P, Borgström F. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int. 2021;32:585–594. doi: 10.1007/s00198-020-05780-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UP_A620_1015/MRC_/Medical Research Council/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- CS-2018-18-ST2-010/DH_/Department of Health/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- MC_PC_21000/MRC_/Medical Research Council/United Kingdom
- MC_PC_21022/MRC_/Medical Research Council/United Kingdom
- MR/P020941/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

