This is a preprint.
Mosaic RBD nanoparticles protect against multiple sarbecovirus challenges in animal models
- PMID: 35378752
- PMCID: PMC8978945
- DOI: 10.1101/2022.03.25.485875
Mosaic RBD nanoparticles protect against multiple sarbecovirus challenges in animal models
Update in
-
Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models.Science. 2022 Aug 5;377(6606):eabq0839. doi: 10.1126/science.abq0839. Epub 2022 Aug 5. Science. 2022. PMID: 35857620 Free PMC article.
Abstract
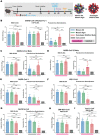
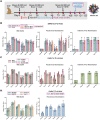
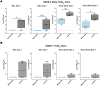
To combat future SARS-CoV-2 variants and spillovers of SARS-like betacoronaviruses (sarbecoviruses) threatening global health, we designed mosaic nanoparticles presenting randomly-arranged sarbecovirus spike receptor-binding domains (RBDs) to elicit antibodies against conserved/relatively-occluded, rather than variable/immunodominant/exposed, epitopes. We compared immune responses elicited by mosaic-8 (SARS-CoV-2 and seven animal sarbecoviruses) and homotypic (only SARS-CoV-2) RBD-nanoparticles in mice and macaques, observing stronger responses elicited by mosaic-8 to mismatched (not on nanoparticles) strains including SARS-CoV and animal sarbecoviruses. Mosaic-8 immunization showed equivalent neutralization of SARS-CoV-2 variants including Omicron and protected from SARS-CoV-2 and SARS-CoV challenges, whereas homotypic SARS-CoV-2 immunization protected only from SARS-CoV-2 challenge. Epitope mapping demonstrated increased targeting of conserved epitopes after mosaic-8 immunization. Together, these results suggest mosaic-8 RBD-nanoparticles could protect against SARS-CoV-2 variants and future sarbecovirus spillovers.
Conflict of interest statement
Competing interests
P.J.B. and A.A.C. are inventors on a US patent application filed by the California Institute of Technology that covers the mosaic nanoparticles described in this work. J.D.B. consults for Moderna and Flagship Labs 77 on topics related to viral evolution. A.J.G, T.N.S., and J.D.B have the potential to receive a share of IP revenue as inventors on a Fred Hutch optioned technology related to deep mutational scanning of viral proteins and RBD-based vaccine formulations.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
