Efficacy and Prognostic Factors for Response to PD-1 Inhibitors in Advanced Cervical Carcinoma: A Retrospective Study
- PMID: 35378925
- PMCID: PMC8976502
- DOI: 10.2147/DDDT.S358302
Efficacy and Prognostic Factors for Response to PD-1 Inhibitors in Advanced Cervical Carcinoma: A Retrospective Study
Abstract
Purpose: Programmed cell death protein 1 (PD-1) inhibitors have shown a therapeutic effect in the treatment of advanced cervical cancer in clinical trials. However, the clinical characteristics associated with response remain undetermined. This study aimed to evaluate the efficacy and prognostic factors of PD-1 inhibitors in patients with advanced cervical cancer in clinical practice.
Patients and methods: The study enrolled patients with recurrent or metastatic cervical cancer treated with PD-1 inhibitors at our center between March 2018 and November 2020. The primary outcomes were the objective response rate (ORR) and progression-free survival (PFS). Secondary endpoints were overall survival (OS) and safety. In addition, independent prognostic factors were identified by multivariate regression analysis.
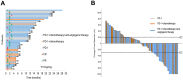
Results: A total of 102 patients were included, and the ORR and disease control rate (DCR) were 51.0% and 66.7%, respectively. Median PFS was 11.0 months (95% CI, 1.7-20.4), while median OS was not achieved. Multivariate analysis indicated that factors associated with a better prognosis (ORR and PFS) included squamous cell carcinoma, a time to recurrence >6 months, and PD-1 plus chemotherapy and anti-angiogenic drugs (p < 0.05). Lines of therapy were independent factors for ORR but not for PFS. We also observed a tendency for longer PFS in patients with lung metastases and lymph node metastases only. Treatment-related adverse events (AEs) were well tolerated and primarily included thrombocytopenia, hepatic dysfunction, anemia, and leukopenia.
Conclusion: PD-1 inhibitors demonstrated beneficial efficacy and safety in advanced cervical cancer, particularly for patients with squamous cell carcinoma, a time to recurrence >6 months, or PD-1 plus chemotherapy and anti-angiogenic drugs. Further studies are needed to confirm the long-term outcomes.
Keywords: PD-1; anti-angiogenic therapy; cervical cancer; chemotherapy; immune checkpoint inhibitors.
© 2022 Cheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. - PubMed
-
- Chen J, Gu W, Yang L, et al. NanoTechnology in the management of cervical cancer. Rev Med Virol. 2015;25(Suppl 1):72–83. - PubMed
-
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

