Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE - Coalition V): A double-blind, multicentre, randomised, controlled trial
- PMID: 35378952
- PMCID: PMC8968238
- DOI: 10.1016/j.lana.2022.100243
Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE - Coalition V): A double-blind, multicentre, randomised, controlled trial
Abstract
Background: Previous Randomised controlled trials (RCT) evaluating chloroquine and hydroxychloroquine in non-hospitalised COVID-19 patients have found no significant difference in hospitalisation rates. However, low statistical power precluded definitive answers.
Methods: We conducted a multicenter, double-blind, RCT in 56 Brazilian sites. Adults with suspected or confirmed COVID-19 presenting with mild or moderate symptoms with ≤ 07 days prior to enrollment and at least one risk factor for clinical deterioration were randomised (1:1) to receive hydroxychloroquine 400 mg twice a day (BID) in the first day, 400 mg once daily (OD) thereafter for a total of seven days, or matching placebo. The primary outcome was hospitalisation due to COVID-19 at 30 days, which was assessed by an adjudication committee masked to treatment allocation and following the intention-to-treat (ITT) principle. An additional analysis was performed only in participants with SARS-CoV-2 infection confirmed by molecular or serology testing (modified ITT [mITT] analysis). This trial was registered at ClinicalTrials.gov, NCT04466540.
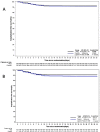
Findings: From May 12, 2020 to July 07, 2021, 1372 patients were randomly allocated to hydroxychloroquine or placebo. There was no significant difference in the risk of hospitalisation between hydroxychloroquine and placebo groups (44/689 [6·4%] and 57/683 [8·3%], RR 0·77 [95% CI 0·52-1·12], respectively, p=0·16), and similar results were found in the mITT analysis with 43/478 [9·0%] and 55/471 [11·7%] events, RR 0·77 [95% CI 0·53-1·12)], respectively, p=0·17. To further complement our data, we conducted a meta-analysis which suggested no significant benefit of hydroxychloroquine in reducing hospitalisation among patients with positive testing (69/1222 [5·6%], and 88/1186 [7·4%]; RR 0·77 [95% CI 0·57-1·04]).
Interpretation: In outpatients with mild or moderate forms of COVID-19, the use of hydroxychloroquine did not reduce the risk of hospitalisation compared to the placebo control. Our findings do not support the routine use of hydroxychloroquine for treatment of COVID-19 in the outpatient setting.
Funding: COALITION COVID-19 Brazil and EMS.
© 2022 The Authors.
Conflict of interest statement
AA reports consultant and lectures fees from Bayer, NovoNordisk and Lilly outside of this submitted work, and research grants from Bayer, EMS Pharma and Population Health Research Institute. GBFO received honoraria for lectures from Novartis and Janssen outside of this submitted work; AD reports lecture fees from Bayer, Libbs, Apsen, and Biolab outside of this submitted work. ASS reports lectures fees from BioLab, Torrents and Servier. AVS reported research grants from AstraZeneca, MSD, Esperion, Clover Biopharm e Enanta Pharmaceutical outside of this submitted work. ABC received honoraria for lectures from EMS Pharma. LCPA reports personal fees from Baxter, Pfizer, and Halex-Istar; and grants from Ache Laboratorios Farmaceuticos, outside of this submitted work. OB reports grants from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Servier, and Amgen, and advisory board and personal fees from Novartis, outside of this submitted work. RBA reports to be an EMS employee. RDL reports grants and personal fees from Bristol Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim, outside of this submitted work. RCR received honoraria for lectures from Novartis outside of this submitted work; VCV reports grants from Aspen Pharma, Pfizer, and Cristalia, outside of this submitted work. The other authors have no conflict to declare.
Figures



References
-
- World Health Organization (WHO) WHO site; 2022. WHO Coronavirus (COVID-19) Dashboard.https://covid19.who.int/ Accessed 11 March 2022.
-
- Callaway E. Beyond Omicron: what's next for COVID's viral evolution. Nature. 2021;600:204–207. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

