Pulsatile Basal Insulin Secretion Is Driven by Glycolytic Oscillations
- PMID: 35378996
- PMCID: PMC9191171
- DOI: 10.1152/physiol.00044.2021
Pulsatile Basal Insulin Secretion Is Driven by Glycolytic Oscillations
Abstract
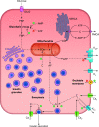
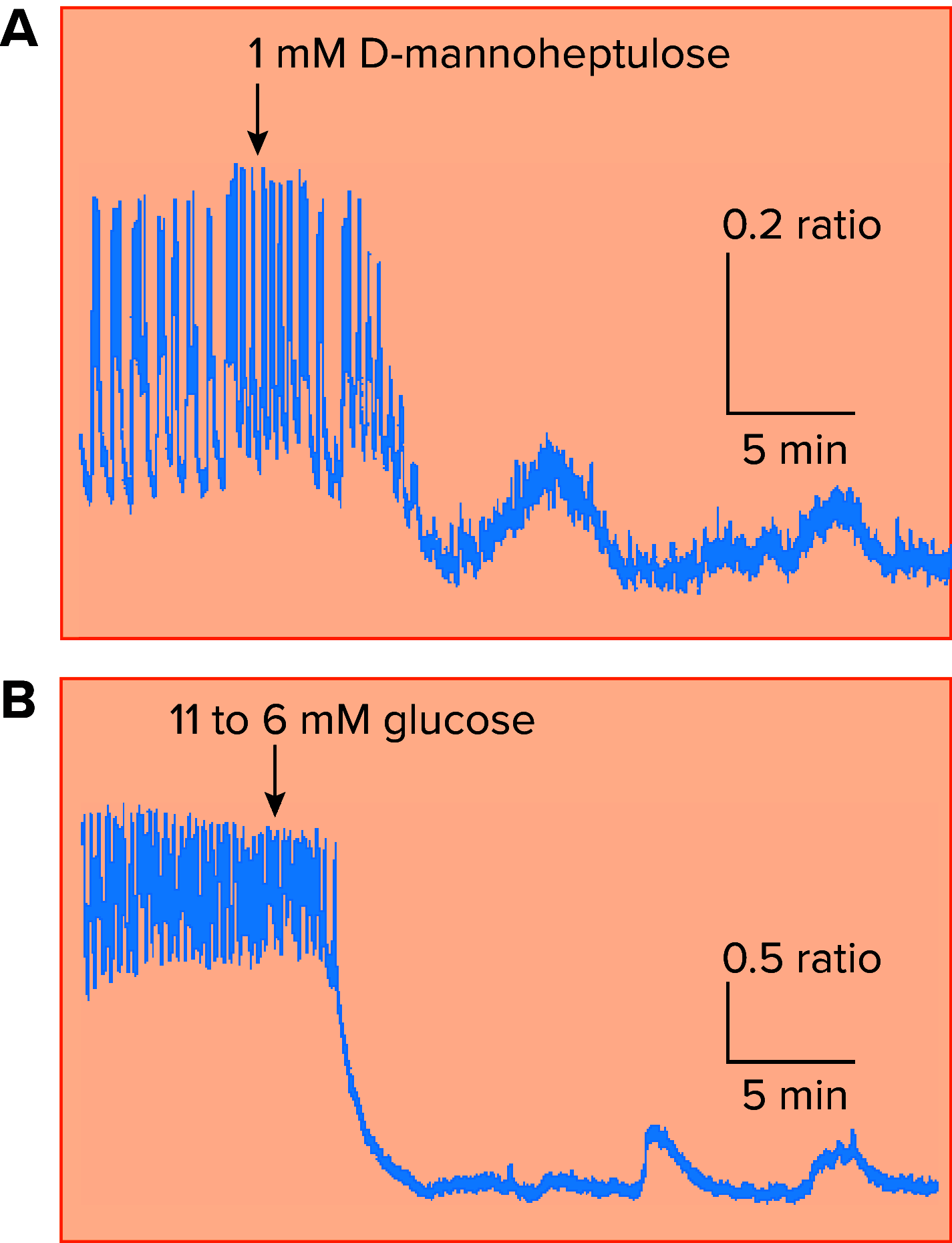
In fasted and fed states, blood insulin levels are oscillatory. While this phenomenon is well studied at high glucose levels, comparatively little is known about its origin under basal conditions. We propose a possible mechanism for basal insulin oscillations based on oscillations in glycolysis, demonstrated using an established mathematical model. At high glucose, this is superseded by a calcium-dependent mechanism.
Keywords: bursting electrical activity; calcium; metabolism; pulsatile insulin secretion.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest 125: 3847–3860, 2015. doi:10.1172/JCI82498. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

