Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications
- PMID: 35378997
- PMCID: PMC9255719
- DOI: 10.1152/physrev.00014.2021
Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications
Abstract
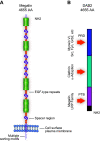
For nearly 50 years the proximal tubule (PT) has been known to reabsorb, process, and either catabolize or transcytose albumin from the glomerular filtrate. Innovative techniques and approaches have provided insights into these processes. Several genetic diseases, nonselective PT cell defects, chronic kidney disease (CKD), and acute PT injury lead to significant albuminuria, reaching nephrotic range. Albumin is also known to stimulate PT injury cascades. Thus, the mechanisms of albumin reabsorption, catabolism, and transcytosis are being reexamined with the use of techniques that allow for novel molecular and cellular discoveries. Megalin, a scavenger receptor, cubilin, amnionless, and Dab2 form a nonselective multireceptor complex that mediates albumin binding and uptake and directs proteins for lysosomal degradation after endocytosis. Albumin transcytosis is mediated by a pH-dependent binding affinity to the neonatal Fc receptor (FcRn) in the endosomal compartments. This reclamation pathway rescues albumin from urinary losses and cellular catabolism, extending its serum half-life. Albumin that has been altered by oxidation, glycation, or carbamylation or because of other bound ligands that do not bind to FcRn traffics to the lysosome. This molecular sorting mechanism reclaims physiological albumin and eliminates potentially toxic albumin. The clinical importance of PT albumin metabolism has also increased as albumin is now being used to bind therapeutic agents to extend their half-life and minimize filtration and kidney injury. The purpose of this review is to update and integrate evolving information regarding the reabsorption and processing of albumin by proximal tubule cells including discussion of genetic disorders and therapeutic considerations.
Keywords: FcRn; cubilin; drug delivery; endocytosis; megalin; transcytosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



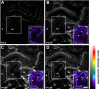
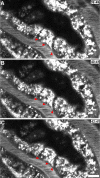
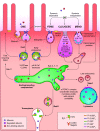

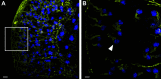



References
-
- Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 27: 482–494, 2016. doi:10.1681/ASN.2014111107. - DOI - PMC - PubMed
-
- Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ. Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24: 1966–1980, 2013. doi:10.1681/ASN.2013010018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

