Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate
- PMID: 35379265
- PMCID: PMC8981610
- DOI: 10.1186/s12974-022-02435-9
Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate
Abstract
Background: Poststroke cognitive impairment (PSCI) is prevalent in stroke patients. The etiology of PSCI remains largely unknown. We previously found that stroke induces gut microbiota dysbiosis which affects brain injury. Hereby, we aimed to investigate whether the gut microbiota contributes to the pathogenesis of PSCI.
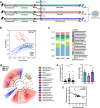
Methods: 83 stroke patients were recruited and their cognitive function were measured by Montreal Cognitive Assessment (MoCA) scores 3 months after stroke onset. The peripheral inflammatory factor levels and gut microbiota compositions of the patients were analyzed. Fecal microbiota transplantation from patients to stroke mice was performed to examine the causal relationship between the gut microbiota and PSCI. The cognitive function of mice was evaluated by Morris water maze test.
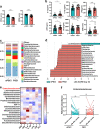
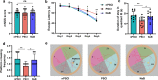
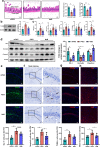
Results: 34 and 49 stroke patients were classified as PSCI and non-PSCI, respectively. Compared with non-PSCI patients, PSCI patients showed significantly higher levels of gut Enterobacteriaceae, lipopolysaccharide (LPS) and peripheral inflammation markers. Consistently, stroke mice that received microbiota from PSCI patients (PSCI mice) presented a higher level of Enterobacteriaceae, intestinal Toll-like receptor-4 (TLR4) expression, circulating LPS, LPS-binding protein (LBP) and inflammatory cytokines, and a lower level of fecal butyrate, severer intestine destruction and cognitive impairment than mice that received microbiota from nPSCI patients (nPSCI mice). In addition, we observed exacerbations in blood-brain barrier (BBB) integrity, microglial activation, neuronal apoptosis in the CA1 region of the hippocampus, and Aβ deposition in the thalamus of PSCI mice in comparison with nPSCI mice. Intraperitoneal injection of LPS after stroke caused similar pathology to those seen in PSCI mice. Supplementation with sodium butyrate (NaB) via drinking water rescued these detrimental changes in PSCI mice.
Conclusions: Our data indicate a cause-effect relationship between gut microbiota and PSCI for the first time, which is likely mediated by inflammation-regulating metabolites including LPS and butyrate.
Keywords: Fecal microbiota transplantation; Hippocampal apoptosis; Lipopolysaccharide; Post-stroke cognitive impairment; β-Amyloid.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Association between post-stroke cognitive impairment and gut microbiota: A PRISMA-compliant systematic review and meta-analysis.Medicine (Baltimore). 2023 Sep 1;102(35):e34764. doi: 10.1097/MD.0000000000034764. Medicine (Baltimore). 2023. PMID: 37657030 Free PMC article.
-
Gut microbiome-derived lipopolysaccharides aggravate cognitive impairment via TLR4-mediated inflammatory signaling in neonatal rats following hypoxic-ischemic brain damage.Brain Behav Immun. 2025 Jul;127:4-24. doi: 10.1016/j.bbi.2025.02.029. Epub 2025 Feb 24. Brain Behav Immun. 2025. PMID: 40010549
-
Association between post-stroke cognitive impairment and gut microbiota in patients with ischemic stroke.Sci Rep. 2025 May 29;15(1):18849. doi: 10.1038/s41598-025-03068-7. Sci Rep. 2025. PMID: 40442236 Free PMC article.
-
[Correlations Between Gut Microbiota Changes and Cognitive Function in Patients with Post-Stroke Cognitive Impairment in the Early Stage].Sichuan Da Xue Xue Bao Yi Xue Ban. 2022 Sep;53(5):857-865. doi: 10.12182/20220960105. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36224689 Free PMC article. Chinese.
-
Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment.Pharmacol Res. 2021 Feb;164:105277. doi: 10.1016/j.phrs.2020.105277. Epub 2020 Nov 7. Pharmacol Res. 2021. PMID: 33166735 Review.
Cited by
-
The contribution of age-related changes in the gut-brain axis to neurological disorders.Gut Microbes. 2024 Jan-Dec;16(1):2302801. doi: 10.1080/19490976.2024.2302801. Epub 2024 Jan 18. Gut Microbes. 2024. PMID: 38237031 Free PMC article. Review.
-
Association between post-stroke cognitive impairment and gut microbiota: A PRISMA-compliant systematic review and meta-analysis.Medicine (Baltimore). 2023 Sep 1;102(35):e34764. doi: 10.1097/MD.0000000000034764. Medicine (Baltimore). 2023. PMID: 37657030 Free PMC article.
-
Update on the mechanism of microglia involvement in post-stroke cognitive impairment.Front Aging Neurosci. 2024 Jun 3;16:1366710. doi: 10.3389/fnagi.2024.1366710. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38887610 Free PMC article. Review.
-
Microbiota-gut-brain-axis: a new target of acupuncture therapy for post-stroke cognitive impairment.Front Microbiol. 2025 Apr 24;16:1425054. doi: 10.3389/fmicb.2025.1425054. eCollection 2025. Front Microbiol. 2025. PMID: 40342596 Free PMC article. Review.
-
Gut-Brain Crosstalk and the Central Mechanisms of Orofacial Pain.Brain Sci. 2023 Oct 13;13(10):1456. doi: 10.3390/brainsci13101456. Brain Sci. 2023. PMID: 37891825 Free PMC article. Review.
References
-
- Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–142. - PubMed
-
- Mok VC, Lam BY, Wong A, Ko H, Markus HS, Wong LK. Early-onset and delayed-onset poststroke dementia—revisiting the mechanisms. Nat Rev Neurol. 2017;13(3):148–159. - PubMed
-
- Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, et al. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(3):199–205. - PubMed
-
- Zheng F, Xie W. High-sensitivity C-reactive protein and cognitive decline: the English Longitudinal Study of Ageing. Psychol Med. 2018;48(8):1381–1389. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

