Predictors of response to intra-arterial vasodilatory therapy of non-occlusive mesenteric ischemia in patients with severe shock: results from a prospective observational study
- PMID: 35379286
- PMCID: PMC8981621
- DOI: 10.1186/s13054-022-03962-w
Predictors of response to intra-arterial vasodilatory therapy of non-occlusive mesenteric ischemia in patients with severe shock: results from a prospective observational study
Abstract
Background: Non-occlusive mesenteric ischemia (NOMI) is a life-threatening condition occurring in patients with shock and is characterized by vasoconstriction of the mesenteric arteries leading to intestinal ischemia and multi-organ failure. Although minimal invasive local intra-arterial infusion of vasodilators into the mesenteric circulation has been suggested as a therapeutic option in NOMI, current knowledge is based on retrospective case series and it remains unclear which patients might benefit. Here, we prospectively analyzed predictors of response to intra-arterial therapy in patients with NOMI.
Methods: This is a prospective single-center observational study to analyze improvement of ischemia (indicated by reduction of blood lactate > 2 mmol/l from baseline after 24 h, primary endpoint) and 28-day mortality (key secondary endpoint) in patients with NOMI undergoing intra-arterial vasodilatory therapy. Predictors of response to therapy concerning primary and key secondary endpoint were identified using a) clinical parameters as well as b) data from 2D-perfusion angiography and c) experimental biomarkers of intestinal injury.
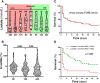
Results: A total of 42 patients were included into this study. At inclusion patients had severe shock, indicated by high doses of norepinephrine (NE) (median (interquartile range (IQR)) 0.37 (0.21-0.60) μg/kg/min), elevated lactate concentrations (9.2 (5.2-13) mmol/l) and multi-organ failure. Patients showed a continuous reduction of lactate following intra-arterial prostaglandin infusion (baseline: (9.2 (5.2-13) mmol/l vs. 24 h: 4.4 (2.5-9.1) mmol/l, p < 0.001) with 22 patients (52.4%) reaching a lactate reduction > 2 mmol/l at 24 h following intervention. Initial higher lactate concentrations and lower NE doses at baseline were independent predictors of an improvement of ischemia. 28-day mortality was 59% in patients with a reduction of lactate > 2 mmol/l 24 h after inclusion, while it was 85% in all other patients (hazard ratio 0.409; 95% CI, 0.14-0.631, p = 0.005).
Conclusions: A reduction of lactate concentrations was observed following implementation of intra-arterial therapy, and lactate reduction was associated with better survival. Our findings concerning outcome predictors in NOMI patients undergoing intra-arterial prostaglandin therapy might help designing a randomized controlled trial to further investigate this therapeutic approach. Trial registration Retrospectively registered on January 22, 2020, at clinicaltrials.gov (REPERFUSE, NCT04235634), https://clinicaltrials.gov/ct2/show/NCT04235634?cond=NOMI&draw=2&rank=1 .
Keywords: Intestinal failure; Non-occlusive mesenteric ischemia; Sepsis; Shock.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- Stahl K, Busch M, Maschke SK, Schneider A, Manns MP, Fuge J, et al. A retrospective analysis of nonocclusive mesenteric ischemia in medical and surgical ICU patients: clinical data on demography, clinical signs, and survival. J Intensive Care Med. 2020;35(11):1162–1172. doi: 10.1177/0885066619837911. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

