Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol
- PMID: 35379348
- PMCID: PMC8981660
- DOI: 10.1186/s13287-022-02815-1
Systematic review and meta-analysis on the use of human platelet lysate for mesenchymal stem cell cultures: comparison with fetal bovine serum and considerations on the production protocol
Abstract
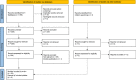
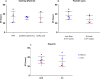
Mesenchymal stem cell (MSC) culturing for cell therapies needs a step forward to be routinely used in clinical settings. Main concerns regard the use of animal origin reagents, in particular supplementing the culture medium with FBS. Lately, Human Platelet Lysate (HPL) has been proposed as animal-free alternative, described as an excellent supplement for culturing MSCs. The aim of this systematic review was to analyze the current literature on the effect of HPL and FBS on ASCs and BMSCs. The primary outcome was the proliferation rate of cells cultured with FBS and HPL. Differences in terms of doubling time (DT) and population doubling (PD) were evaluated by meta-analysis, subgrouping data according to the cell type. A total of 35 articles were included. BMSCs and ASCs were used in 65.7% (23) and 28.6% (10) studies, respectively. Only two studies included both cell types. Overall, 22 studies were eligible for the meta-analysis. Among them, 9 articles described ASCs and 13 BMSCs. The results showed that BMSCs and ASCs cultured with 10% HPL and 5% HPL have lower DT and higher PD compared to cells cultured with 10% FBS. A possible correlation between the DT decrease and the application of at least 3 freeze/thaw cycles to induce platelet lysis was found. Additionally, HPL increased VEGF secretion and maintained the immuno-modulatory abilities for both cell types. The clarification reported here of the higher efficiency of HPL compared to FBS can help the transition of the scientific community towards clinical-related procedures. 1. The meta-analysis shows that HPL induces a population doubling increase and a doubling time decrease of both ASCs and BMSCs compared to FBS. 2. When at least 3 freeze/thaw cycles are applied to induce platelet lysis, the doubling time of HPL-cultured cells is lower than FBS-cultured cells (Created with BioRender.com).
Keywords: Cell proliferation; Fetal bovine serum (FBS); Freeze/thaw cycles; Human platelet lysate (HPL); Mesenchymal stem cells (MSC).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Human platelet lysate produced from leukoreduction filter contents enables sufficient MSC growth.Stem Cell Res Ther. 2025 Apr 23;16(1):205. doi: 10.1186/s13287-025-04329-y. Stem Cell Res Ther. 2025. PMID: 40270024 Free PMC article.
-
Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2018 Mar 22;9(1):75. doi: 10.1186/s13287-018-0823-3. Stem Cell Res Ther. 2018. PMID: 29566772 Free PMC article.
-
Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: a systematic review.J Transl Med. 2020 Sep 15;18(1):351. doi: 10.1186/s12967-020-02489-4. J Transl Med. 2020. PMID: 32933520 Free PMC article.
-
Bioactive human platelet lysate gel for enhanced proliferation of human umbilical cord tissue derived mesenchymal stem cells.Cell Tissue Bank. 2025 May 14;26(2):25. doi: 10.1007/s10561-025-10175-2. Cell Tissue Bank. 2025. PMID: 40366481
-
Feasibility of Human Platelet Lysate as an Alternative to Foetal Bovine Serum for In Vitro Expansion of Chondrocytes.Int J Mol Sci. 2021 Jan 28;22(3):1269. doi: 10.3390/ijms22031269. Int J Mol Sci. 2021. PMID: 33525349 Free PMC article. Review.
Cited by
-
Increasing the biomolecular relevance of cell culture practice.J Biomed Sci. 2025 Jan 3;32(1):3. doi: 10.1186/s12929-024-01095-6. J Biomed Sci. 2025. PMID: 39748368 Free PMC article. Review.
-
Media matters: culture medium-dependent hypervariable phenotype of mesenchymal stromal cells.Stem Cell Res Ther. 2023 Dec 12;14(1):363. doi: 10.1186/s13287-023-03589-w. Stem Cell Res Ther. 2023. PMID: 38087388 Free PMC article.
-
Therapeutic potential of adipose derived stromal cells for major skin inflammatory diseases.Front Med (Lausanne). 2024 Feb 23;11:1298229. doi: 10.3389/fmed.2024.1298229. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38463491 Free PMC article. Review.
-
Review: progression and heterogeneity of multiple sources of mesenchymal stromal cells for the treatment of rheumatoid arthritis.Stem Cell Res Ther. 2025 Jul 9;16(1):358. doi: 10.1186/s13287-025-04515-y. Stem Cell Res Ther. 2025. PMID: 40629483 Free PMC article.
-
Evaluation of a Serum-Free Medium for Human Epithelial and Stromal Cell Culture.Int J Mol Sci. 2022 Sep 2;23(17):10035. doi: 10.3390/ijms231710035. Int J Mol Sci. 2022. PMID: 36077429 Free PMC article.
References
-
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. - PubMed
-
- Schallmoser K, Henschler R, Gabriel C, Koh MBC, Burnouf T. Production and quality requirements of human platelet lysate: a position statement from the working party on cellular therapies of the international society of blood transfusion. Trends Biotechnol. 2020;38(1):13–23. - PubMed
-
- Doucet C, Ernou I, Zhang Y, Llense J-R, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

